Fused heteroaromatic organic luminescent material and its preparation method and application
A luminescent material and organic technology, applied in luminescent materials, material analysis through optical means, organic chemistry, etc., to achieve high yield, overcome low luminous efficiency, and large molar absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] N11-(Diethylamino)benzo[g]benzo[4,5]imidazo[1,2-a][1,8]naphthyridine-6-carbonitrile (NBC)
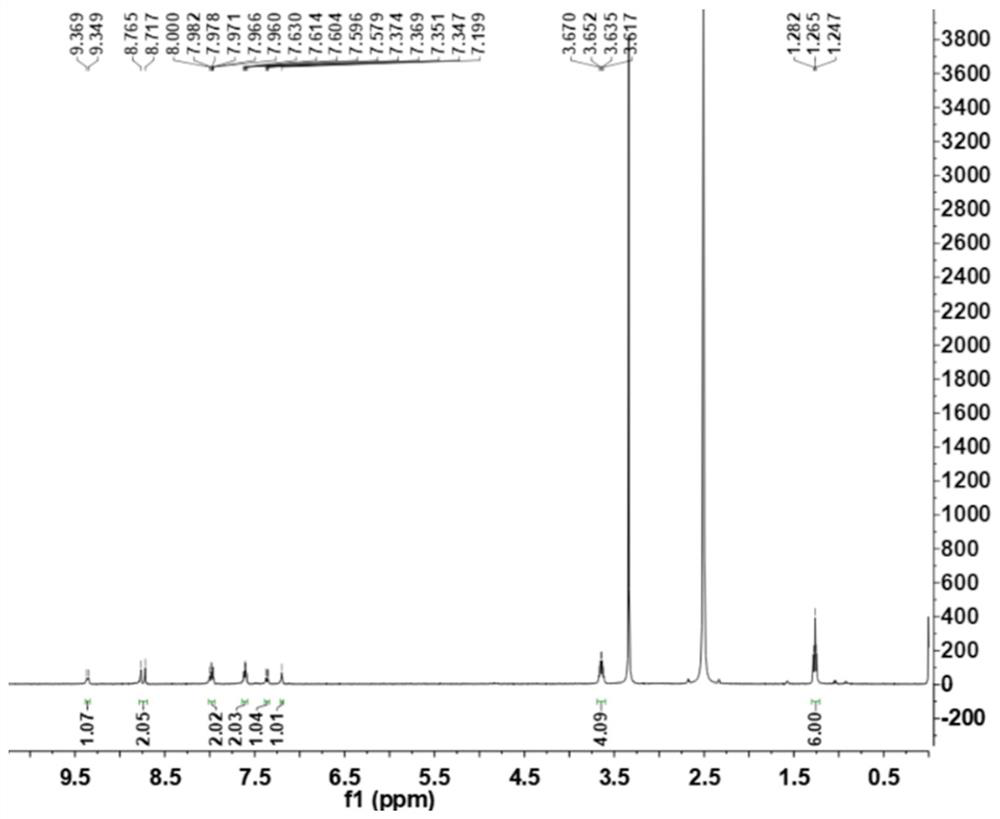
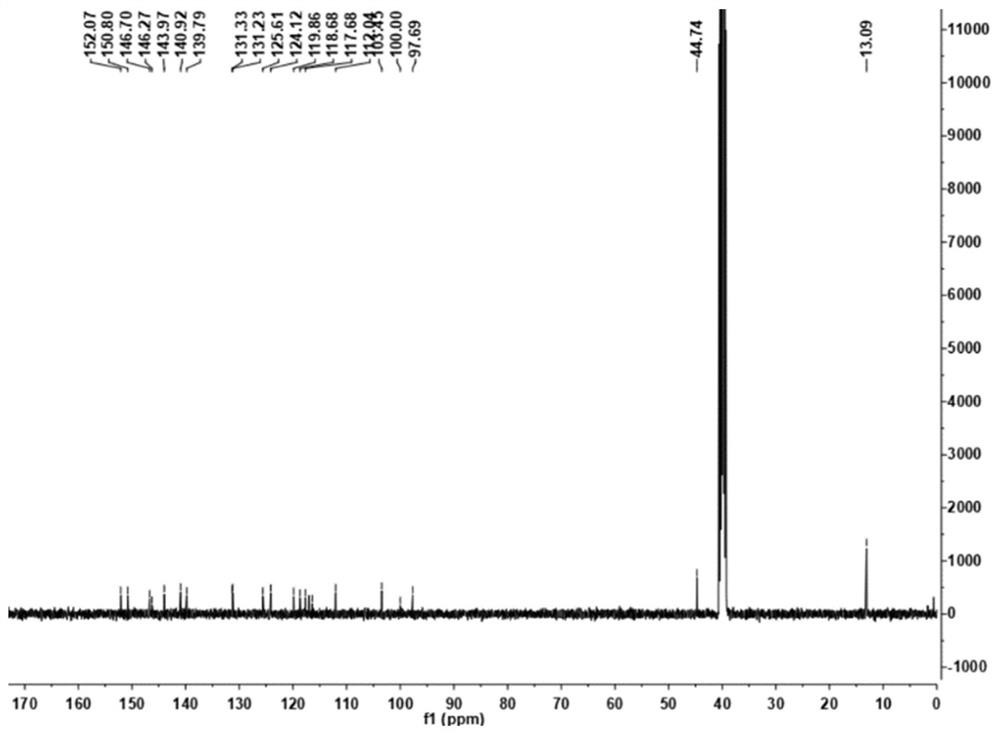
[0036] Add 1.00 g (3.8 mmol) of 2-chloro-7-(diethylamino) quinoline-3-carbaldehyde, 0.50 g (3.2 mmol) of 2-cyanomethylbenzimidazole and N,N- Add 10 mL of dimethylformamide, then add 0.5 mL of pyridine, heat and stir. The resulting mixture was then stirred at 85 °C until the reaction was complete (checked by TLC). Naturally cooled to room temperature, 30 mL of water was added to the reaction solution, and an orange solid was precipitated, filtered under reduced pressure, silica gel column chromatography, and eluted with petroleum ether:ethyl acetate=5:1 to obtain 1.05 g of an orange solid with a yield of 70 %. 1 H NMR (400MHz, DMSO-d6) δ: 9.36 (d, J = 8.0Hz, 1H), 8.74 (d, J = 23.2Hz, 1H), 8.02-7.93 (m, 2H), 7.65–7.57 (m, 2H), 7.36(dd, J=9.2, 2.0Hz, 1H), 7.20(s, 1H), 3.64(q, J=7.2Hz, 4H), 1.26(t, J=6.8Hz, 6H). 13 C NMR(100MHz,DMSO)δ:152.07,150.80,146.70,146.27,143.97,140.92,139...
Embodiment 2
[0039] The preparation of embodiment 2 test solutions
[0040] (1) Preparation procedure of stock solution:
[0041] Weigh the NBCs prepared in Example 1 and add them into 10mL colorimetric tubes to prepare a concentration of 1.0×10 -3mol / L chloroform solution was used as the stock solution, and then the NBC stock solution was diluted 10, 100, 1000, and 10000 times to prepare a concentration of 1.0×10 -4 mol / L, 1.0×10 -5 mol / L, 1.0×10 -6 mol / L, 1.0×10 -7 mol / L chloroform solution. Take 10 μL concentration as 1.0×10 -5 Add the mol / L NBC solution into twelve 10mL colorimetric tubes, dilute to 10mL with different solvents, and make a concentration of 1.0×10 -6 mol / L solution to measure the fluorescence quantum yield of NBC, with rhodamine B as the standard reference.
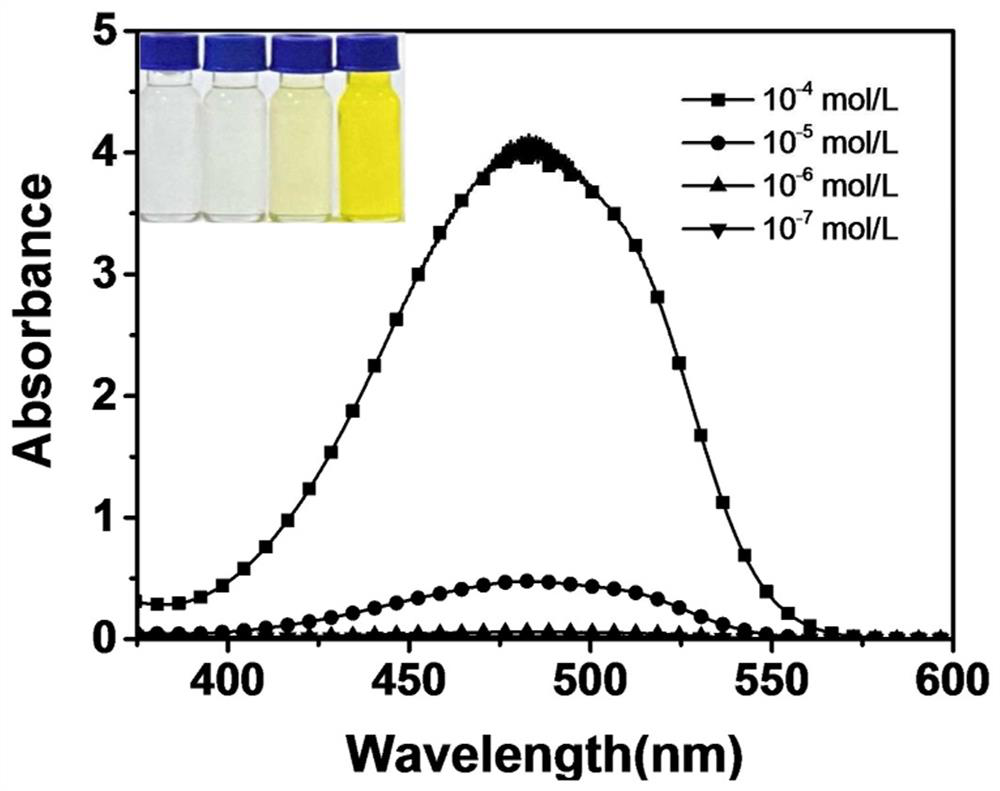
[0042] (2) NBC concentration-dependent test:
[0043] Take 2mL NBC chloroform solutions with different concentrations to test the UV-Vis absorption spectrum and fluorescence emission spectrum. Such as im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com