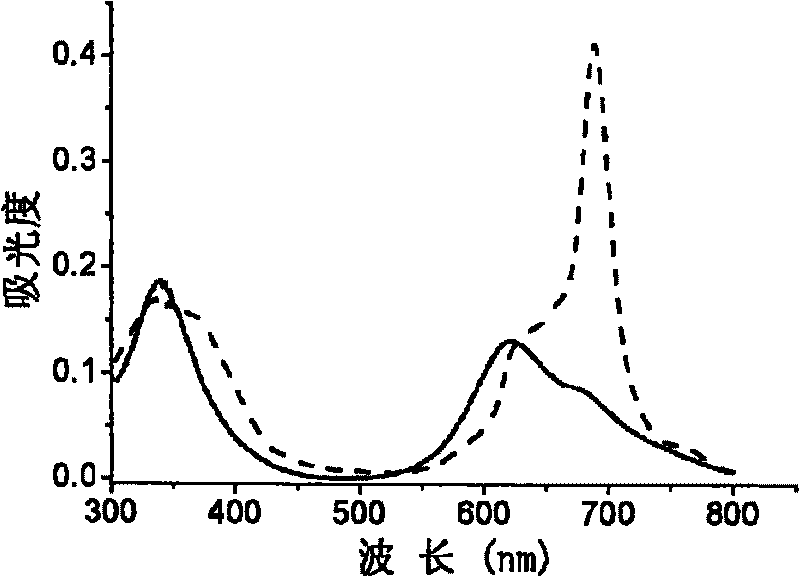
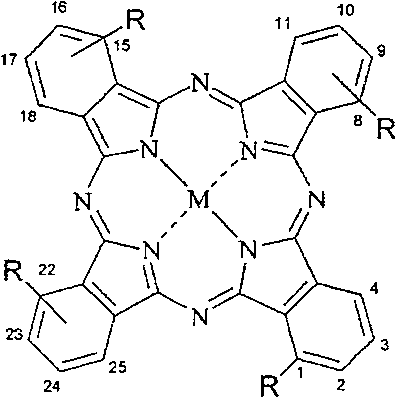
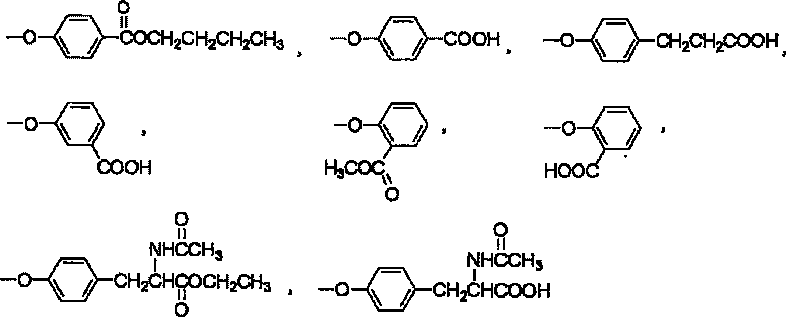
Use of non-periphery substituted phthalocyaniu metal complex
A metal complex, non-peripheral technology, applied in the field of non-peripheral substituted phthalocyanine metal complexes, can solve problems such as difficult aggregation, and achieve the effects of difficult aggregation, high photodynamic activity, and improved photodynamic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The steps of the preparation method of the non-peripheral substituted phthalocyanine metal complex of structural formula (1) are:
[0042] (1): With 3-nitrophthalonitrile and the alcohol derivative of the substituting group described in claim 1 as the reactant, the molar ratio of the two is 1: 0.8-1.2; the alcohol derivative is butylparaben, 4-hydroxybenzoic acid, 3-(4-hydroxyphenyl)propionic acid, 3-hydroxybenzoic acid, methyl salicylate, ethyl N-acetyltyrosine, or N-acetyl One of the base tyrosine;
[0043] (2): With dimethyl sulfoxide or N, N-dimethylformamide as solvent, the amount of solvent needs 3-8ml for every mmol reactant 3-nitrophthalonitrile, in the presence of potassium carbonate and nitrogen Under the protection of , react at room temperature-60 ℃ for 8-24 hours, obtain intermediate substituted phthalonitrile, described substituted phthalonitrile is shown in formula (7), R and claim 1 described substituting group R same;
[0044] (3): Using the intermed...
Embodiment 1
[0077] Synthesis of 1,8(11), 15(18), 22(25)-tetrakis(4-butoxycarbonylphenoxy)phthalocyanine zinc(II)
[0078] This compound can also be called four-a-(4-butoxycarbonylphenoxy)zinc phthalocyanine (II), and its structure is shown in formula 1, wherein:
[0079]
[0080] (1) Preparation of intermediate 3-(4-butoxycarbonylphenoxy) phthalonitrile:
[0081] Under the protection of nitrogen, 5mmol butylparaben and 4-6mmol (preferably 5mmol) 3-nitrophthalonitrile were added to 15-40ml (preferably 20ml) dimethyl sulfoxide (DMSO) or N, N - In dimethylformamide (DMF), after stirring at room temperature for 10 minutes, add 1.5 g (10.9 mmol) of anhydrous potassium carbonate, and react for 12 to 24 hours (preferably 16 hours). The reaction mixture was added to 200ml of ice-water mixture, stirred to precipitate a large amount of white precipitate, left to stand, filtered by suction, washed with water until neutral, the filter cake was collected, dried under normal pressure at 70°C to obt...
Embodiment 2
[0088] Synthesis of 1,8(11), 15(18), 22(25)-tetrakis(4-carboxyphenoxy)phthalocyanine zinc(II)
[0089] This compound can also be called four-a-(4-carboxyphenoxy) zinc phthalocyanine (II), and its structure is shown in formula 1, wherein:
[0090]
[0091] (1) Preparation of intermediate 3-(4-carboxyphenoxy) phthalonitrile:
[0092] Under the protection of nitrogen, 0.69g (5mmol) 4-hydroxybenzoic acid, 0.87g (5mmol) 3-nitrophthalonitrile were dissolved in 20ml dimethyl sulfoxide (DMSO), after stirring at room temperature for 10 minutes, add 1.5 g (10.9 mmol) of anhydrous potassium carbonate, after 10 minutes, add 1.0 g of potassium carbonate, and continue the reaction for 12 to 24 hours (preferably 14 hours). Suction filter the reaction mixture, collect the filtrate, add 200ml ice-water mixture to the filtrate, adjust the pH value to 1~3 (preferably 2) with 2mol / L HCl solution, the product is precipitated out, filter, wash with water until neutral, collect The filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com