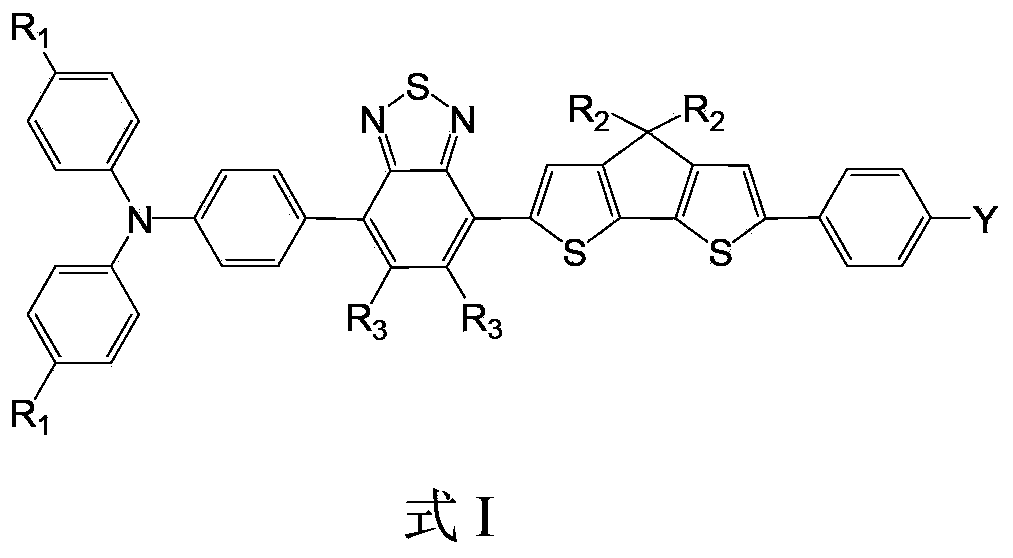
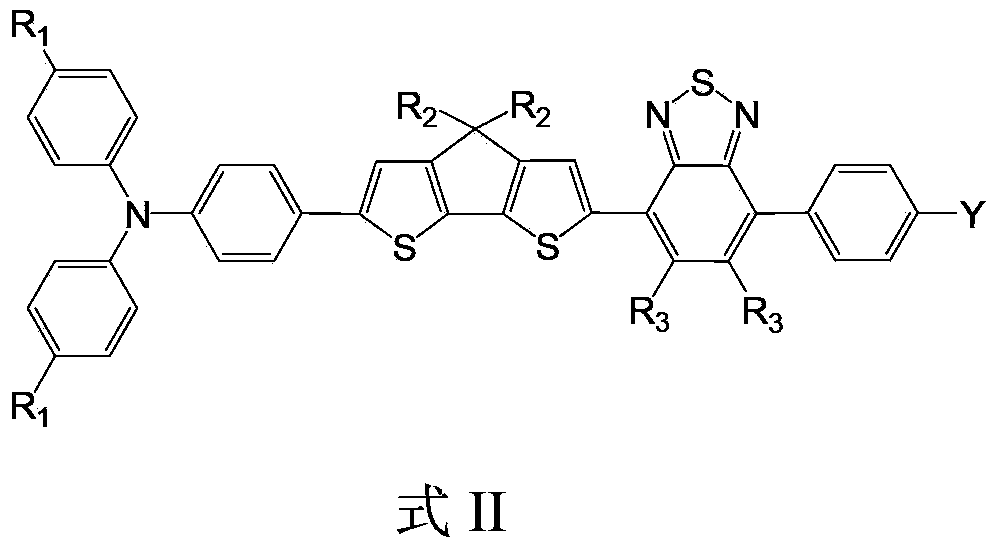
Organic dye containing benzothiadiazole-thienocyclopenta and applications of organic dye in dye-sensitized solar cells
A technology of benzothiadiazole and organic dyes, applied in organic dyes, azo dyes, photovoltaic power generation and other directions, can solve the problem of poor light absorption ability, achieve high molar absorption coefficient, wide spectral response range, narrow molecular energy gap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the preparation of organic dye III
[0018] The synthetic route is as follows:
[0019]
[0020] The raw materials 1 and 4 used in this example, as well as other reagents can be obtained commercially. Raw material 7 was prepared according to literature Li, R.; Liu, J.; Cai, N.; Zhang, M.; Wang, P.J.Phys.Chem.B 2010,114,4461.
[0021] Synthesis of Intermediate 2:
[0022] Add 5.50 g of raw material 1 and 50 ml of n-butanol into a 100 ml round bottom flask, slowly add 1.5 ml of concentrated sulfuric acid dropwise, heat the reaction system under reflux for 20 hours and cool to room temperature, add 50 ml of water to the reaction system, and wash with chloroform (3×50 ml) extracted three times, combined the organic phases, dried with anhydrous sodium sulfate and filtered, and the crude product obtained after concentrating the filtrate was passed through a silica gel column with ethyl acetate / petroleum ether (volume ratio 1 / 20) as eluent After chromatogra...
Embodiment 2
[0062] Embodiment 2: the preparation of organic dye IV
[0063] The synthetic route is as follows:
[0064]
[0065] Raw material 12 was prepared according to literature Li, R.; Liu, J.; Cai, N.; Zhang, M.; Wang, P.J.Phys.Chem.B 2010, 114, 4461.
[0066] The synthesis of intermediates 3 and 5 was obtained with reference to the preparation method in Example 1;
[0067] Synthesis of intermediate 11:
[0068] Add 4 grams of intermediate 3, 4.6 grams of 4,7-dibromobenzothiadiazole, 16.4 grams of sodium carbonate and 280 milliliters of ethanol / benzene / water mixed solvent (volume ratio 1 / 4 / 2) in a 500 ml round bottom flask ), under the protection of argon, add 0.3 g of tetrakistriphenylphosphopalladium, heat the reaction system to reflux for 2 hours, then cool to room temperature, add 200 ml of water, extract three times with chloroform (3×100 ml), combine the organic phases, and use After drying with anhydrous sodium sulfate and filtering, the crude product obtained after con...
Embodiment 3
[0093] Nano TiO 2 The structural double-layer membrane electrode was immersed in acetonitrile / tert-butanol (volume ratio, 1:1) solution containing 100 micromoles per liter of dye III for 12 hours, and then the glass electrode spin-coated with nano-platinum was passed through a 35 microns thick Hot melt ring and TiO 2 The structural double-layer film electrode is heated and sealed, and finally the electrolyte is injected into the gap between the two electrodes, which constitutes a dye-sensitized solar cell. The performance structure of the dye-sensitized solar cells obtained by the test is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com