Quinoxaline-based conjugated polymer, and preparation method and application thereof in polymer solar batteries
A technology of conjugated polymers and alkyl groups, which is applied in the field of quinoxaline conjugated polymers and its preparation and its application in polymer solar cells. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
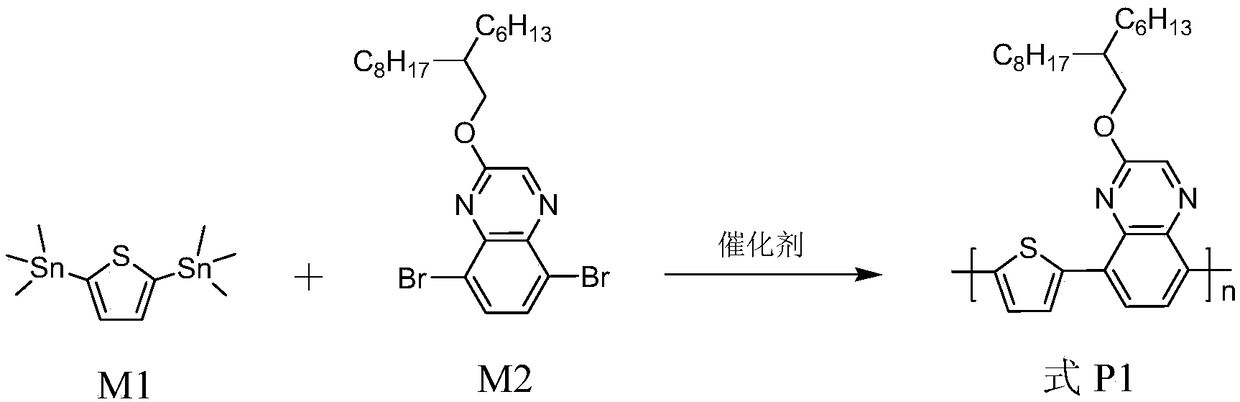
[0085] The synthesis of polymer shown in embodiment 1, formula P1
[0086] Its reaction equation is as figure 1 shown. Concrete reaction steps and reaction conditions are as follows:
[0087]
[0088] Take 0.3mmol each of monomers M1 and M2, dissolve them in a mixed solvent of toluene (8mL) and DMF (2mL), exhaust the air with argon for 5 minutes, and then add the catalyst tetrakis(triphenylphosphine)palladium(0) (20mg, 0.017mmol) continued to evacuate the air for 25 minutes, and then stopped the reaction at the reflux temperature of toluene for 32 hours. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally, it was dissolved in chloroform, precipitated into methanol, filtered, and vacuum-dried for one day to obtain a polymer represented by formula P1 as a red solid powder with a yield of 95%.
[0089] Structu...
Embodiment 2
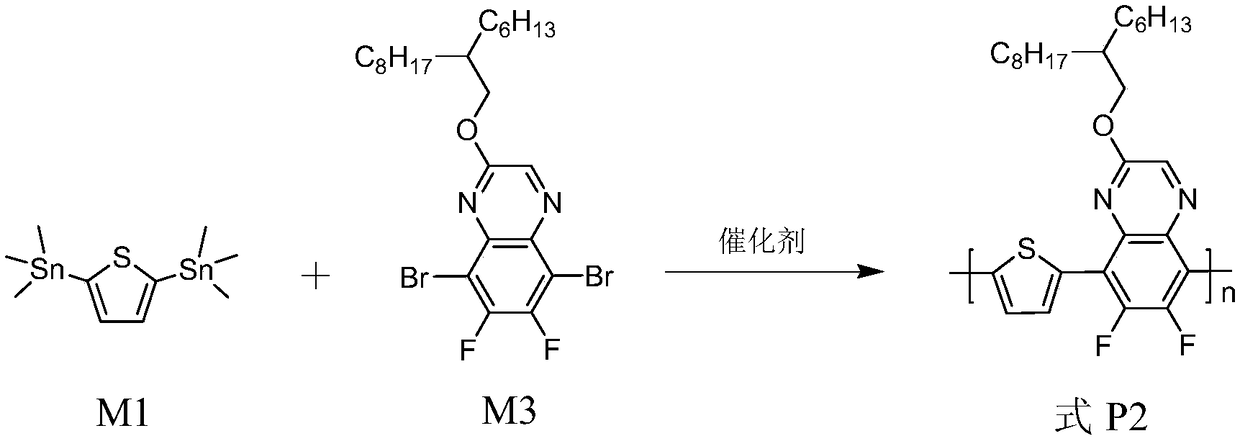
[0094] The synthesis of polymer shown in embodiment 2, formula P2
[0095] Its reaction equation is as figure 2 shown. Concrete reaction steps and reaction conditions are as follows:
[0096]
[0097] Take 0.3 mmol each of monomers M1 and M3, dissolve them in a mixed solvent of toluene (8 mL) and DMF (2 mL), exhaust the air with argon for 5 minutes, and then add the catalyst tetrakis(triphenylphosphine)palladium(0) (20mg, 0.017mmol) continued to evacuate the air for 25 minutes, and then stopped the reaction at the reflux temperature of toluene for 32 hours. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally, it was dissolved in chloroform, precipitated into methanol, filtered, and vacuum-dried for one day to obtain a polymer represented by formula P2 as a red solid powder with a yield of 96%.
[0098] Str...
Embodiment 3
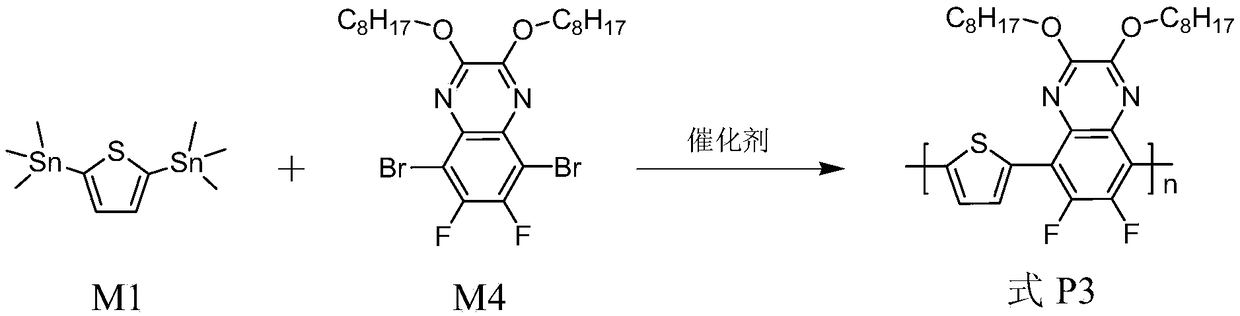
[0103] The synthesis of polymer shown in embodiment 3, formula P3
[0104] Its reaction equation is as image 3 shown. Concrete reaction steps and reaction conditions are as follows:
[0105]
[0106] Take 0.3mmol each of monomers M1 and M4, dissolve them in a mixed solvent of toluene (8mL) and DMF (2mL), exhaust the air with argon for 5 minutes, and then add the catalyst tetrakis(triphenylphosphine)palladium(0) (20mg, 0.017mmol) continued to evacuate the air for 25 minutes, and then stopped the reaction at the reflux temperature of toluene for 32 hours. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally, it was dissolved in chloroform, precipitated into methanol, filtered, and vacuum-dried for one day to obtain a polymer represented by formula P3 as a red solid powder with a yield of 97%.
[0107] The obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com