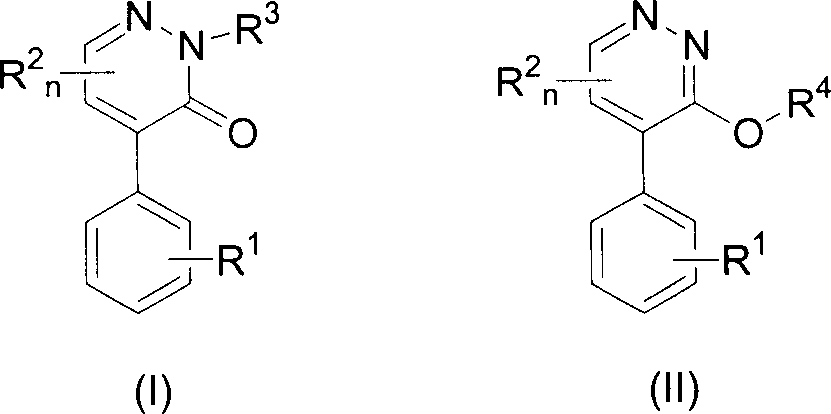
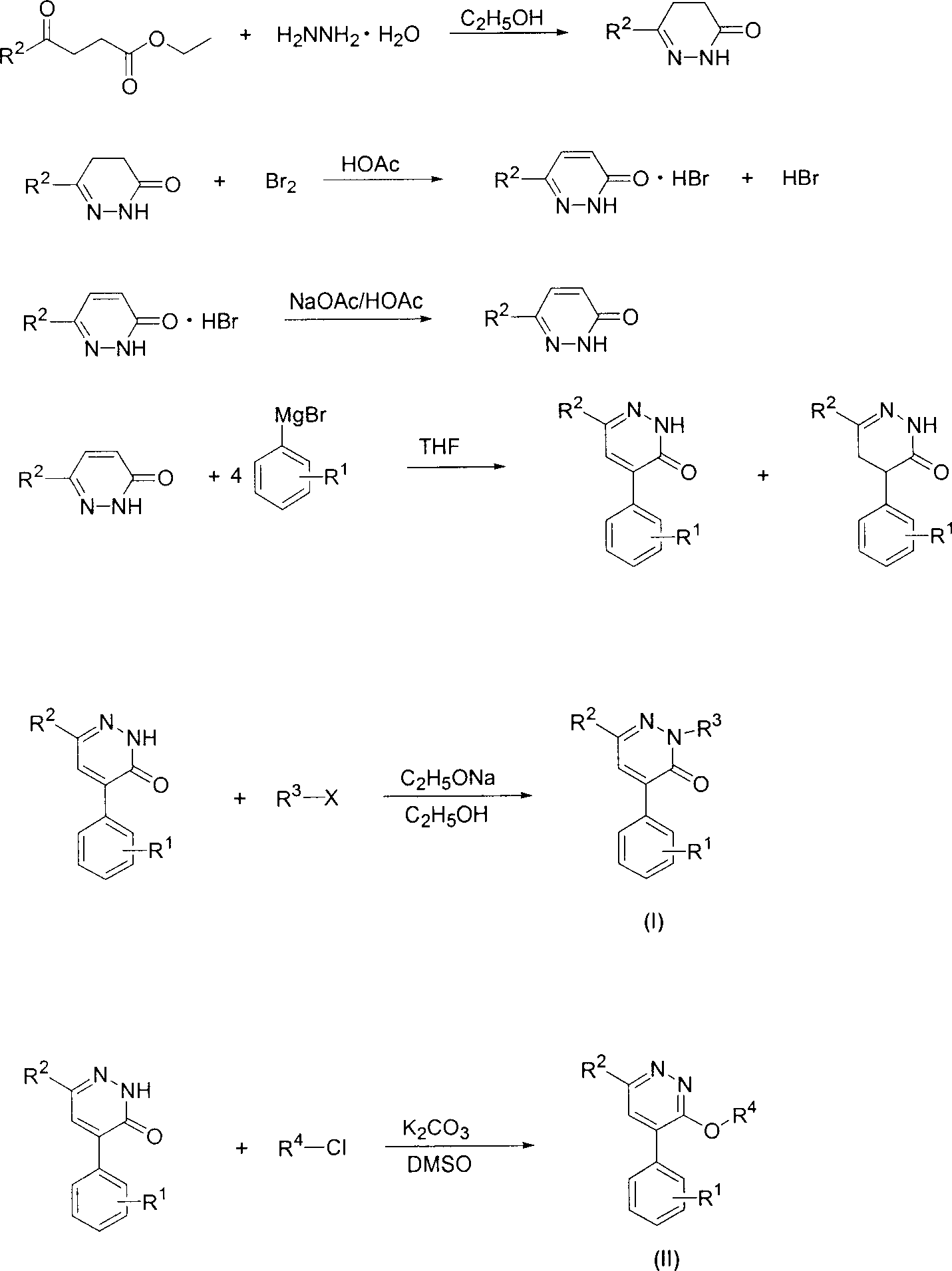4-substituted phenyl pyridazine compound and herbicidal activity
A compound and substituent technology, applied in the field of 4-substituted phenylpyridazine compounds and herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: 4,5-dihydro-6-methyl-3-pyridazinone
[0036] 42.3 g of ethyl levulinate and 27.9 g of hydrazine hydrate were mixed, and a small amount of ethanol was added to obtain a homogeneous solution. The mixture was heated to boiling, refluxed for 1 hour, cooled to room temperature, and left overnight to obtain colorless flaky crystals of 4,5-dihydro-6-methyl-3-pyridazinone with a yield of 96%.
Embodiment 2
[0037] Example 2: 6-methyl-3-pyridazinone
[0038] Dissolve 55g of 4,5-dihydro-6-methyl-3-pyridazinone in 120ml of freshly steamed glacial acetic acid, slowly drop in 33ml of liquid bromine, heat to reflux after dripping, and place it overnight after reflux for 15min. The reaction solution was suction filtered to obtain a complex of 6-methyl-3-pyridazinone and hydrogen bromide. Dissolve 22.4 g of the obtained complex of 6-methyl-3-pyridazinone and hydrogen bromide in 85 ml of glacial acetic acid, add 13.5 g of anhydrous sodium acetate, heat the mixture to boiling, and reflux for 1 h. The solvent was distilled off under reduced pressure, and the residue was added to ice water to obtain a white solid, which was filtered with suction and dried to obtain 12.9 g of a white solid with a yield of 80%.
Embodiment 3
[0039] Example 3: 4-(3-trifluoromethylphenyl)-6-methyl-3-pyridazinone
[0040] 10 mmol of 6-methyl-3-pyridazinone was dissolved in 50 ml of anhydrous tetrahydrofuran, and 40 mmol of 3-trifluoromethylphenylmagnesium bromide in tetrahydrofuran was added at room temperature. The mixture was heated to boiling, refluxed for 5 hours and then hydrolyzed with saturated ammonium chloride solution. The organic phase was washed with saturated sodium chloride solution, dried, spin-dried and separated by column chromatography. The eluent was ethyl acetate:petroleum ether , to give 4-(3-trifluoromethylphenyl)-6-methyl-3-pyridazinone and 4,5-dihydro-4-(3-trifluoromethylphenyl)-6-methyl -3-pyridazinone, the yields were 38.58% and 16.89%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com