Thiazole schiff base containing nitryl, preparation and uses thereof
A technology of thiazole schiff base and schiff base, applied in the field of new compounds and their preparation, can solve the problems of no research report on synthesis and anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
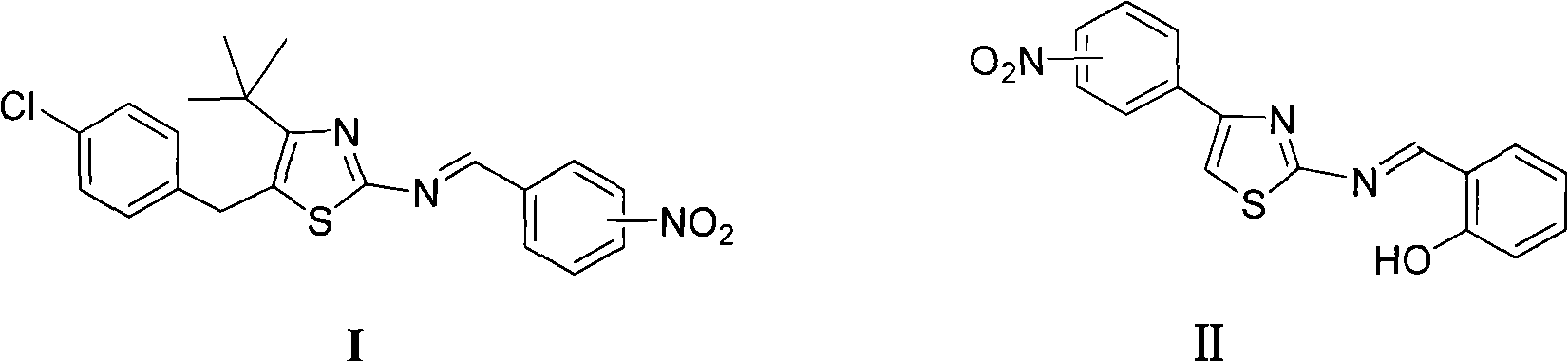
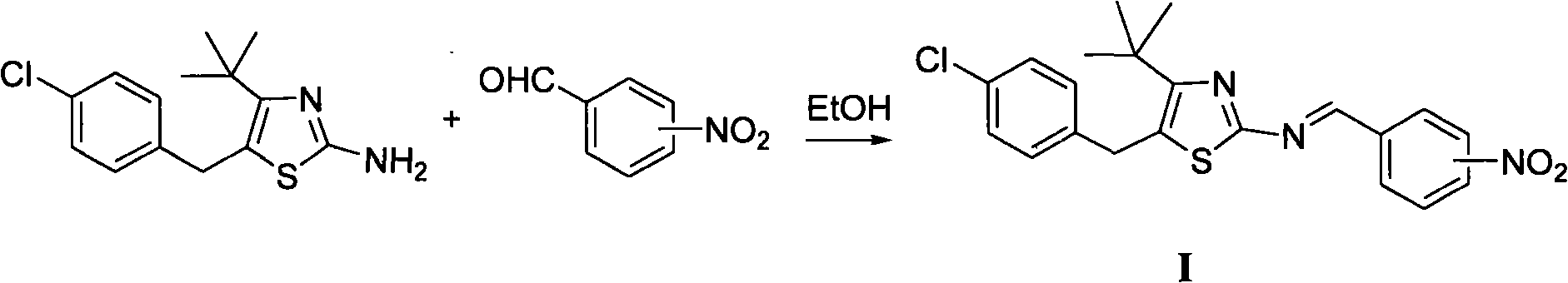
[0014] Synthesis of Example 15-(4-chlorobenzyl)-4-tert-butyl-2-(2-nitrobenzimino)thiazole (Ia)
[0015]
[0016] 1mmol 2-nitrobenzaldehyde, 20mL ethanol, slowly add 1mmol 5-(4-chlorobenzyl)-4-tert-butyl-2-aminothiazole ethanol solution dropwise under stirring at room temperature, and reflux for 2h after addition to obtain 5-( 4-Chlorobenzyl)-4-tert-butyl-2-(2-nitrobenzimino)thiazole, yield 72.6%, melting point: 105.1~106.9°C. 1 H NMR (400MHz, CDCl 3 )δ: 1.42(s, 9H, (CH 3 ) 3 ), 4.26 (s, 2H, CH 2 ), 7.14 (d, J=8.0Hz, 2H, C 6 h 4 Cl 3,5-H), 7.26 (d, J=8.0Hz, 2H, C 6 h 4 Cl2, 6-H), 7.62 (t, J=8.0Hz, 1H, C 6 h 3 5-H), 7.76(t, J=8.0Hz, 1H, C 6 h 3 4-H), 8.02(d, J=8.0Hz, 1H, C 6 h 3 6-H), 8.35(dd, J=8.0Hz, J=4.0Hz, 1H, C 6 h 3 3-H), 9.25 (s, 1H, N=CH).
Embodiment 25
[0017] Synthesis of Example 25-(4-chlorophenylbenzyl)-4-tert-butyl-2-(4-nitrobenzyl imino)thiazole (Ib)
[0018]
[0019] Add 1mmol 4-nitrobenzaldehyde and 20mL ethanol to a four-neck flask, slowly add 1mmol ethanol solution of 5-(4-chlorobenzyl)-4-tert-butyl-2-aminothiazole dropwise under stirring at room temperature, and the addition is complete. Refluxing reaction for 2 hours gave 5-(4-chlorophenylbenzyl)-4-tert-butyl-2-(4-nitrobenzimino)thiazole with a yield of 84.7% and a melting point of 127.8-128.2°C. 1 H NMR (400MHz, CDCl 3 )δ: 1.43(s, 9H, (CH 3 ) 3 ), 4.27 (s, 2H, CH 2 ), 7.14 (d, J=8.4Hz, 2H, C 6 h 4 Cl3, 5-H), 7.28 (d, J=8.4Hz, 2H, C 6 h 4 Cl 2,6-H), 8.08(d, J=8.8Hz, 2H, C 6 h 4 NO 2 3,5-H), 8.28(d, J=8.8Hz, 2H, C 6 h 4 NO 2 2,6-H), 8.89 (s, 1H, N=CH).
Embodiment 35
[0020] Synthesis of Example 35-(4-chlorobenzyl)-4-tert-butyl-2-(2-chloro-5-nitrobenzyl imino)thiazole (Ic)
[0021]
[0022] Add 1mmol 2-chloro-5--nitrobenzaldehyde and 20mL ethanol to a four-neck flask, slowly add 1mmol 5-(4-chlorobenzyl)-4-tert-butyl-2-aminothiazole dropwise under stirring at room temperature Ethanol solution, after adding, reflux reaction for 2 hours to get 5-(4-chlorobenzyl)-4-tert-butyl-2-(2-chloro-5-nitrobenzimino)thiazole, yield 42.5%, melting point : 99.2~102.3°C. 1 HNMR (400MHz, CDCl 3 )δ: 1.44(s, 9H, (CH 3 ) 3 ), 4.28 (s, 2H, CH 2 ), 7.14 (d, J=8.4Hz, 2H, C 6 h 4 3,5-H), 7.28 (d, J=8.4Hz, 2H, C 6 h 4 2,6-H), 7.58 (d, J=8.4Hz, 1H, C 6 h 3 3-H), 8.22(dd, J=8.4Hz, J=2.8Hz, 1H, C 6 h 3 4-H), 9.11(d, J=2.8Hz, 1H, C 6 h 3 6-H), 9.24 (s, 1H, N=CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com