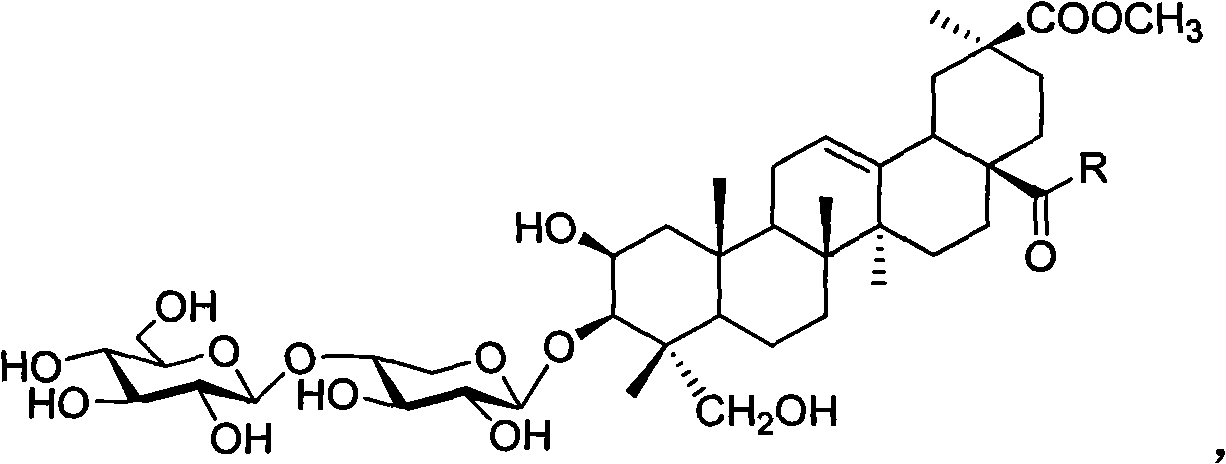
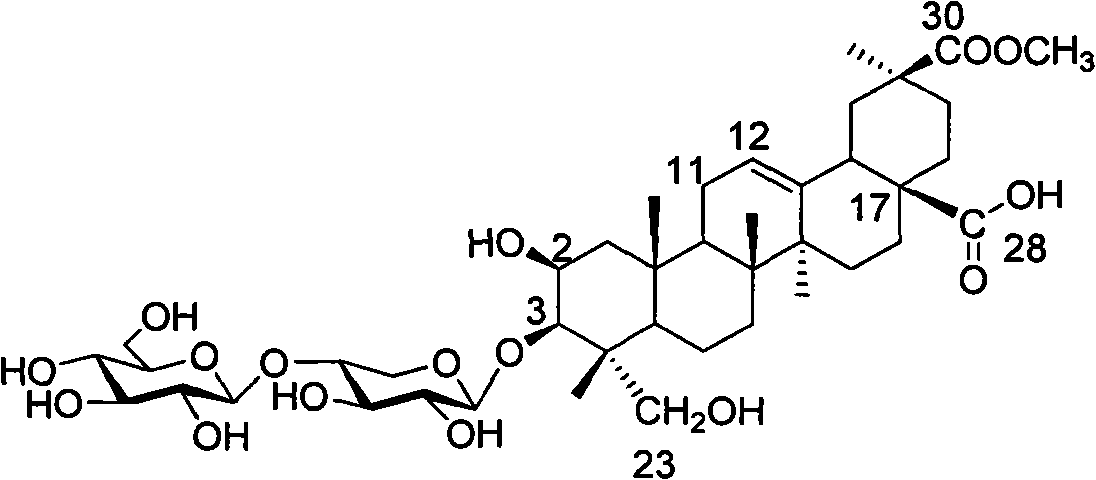
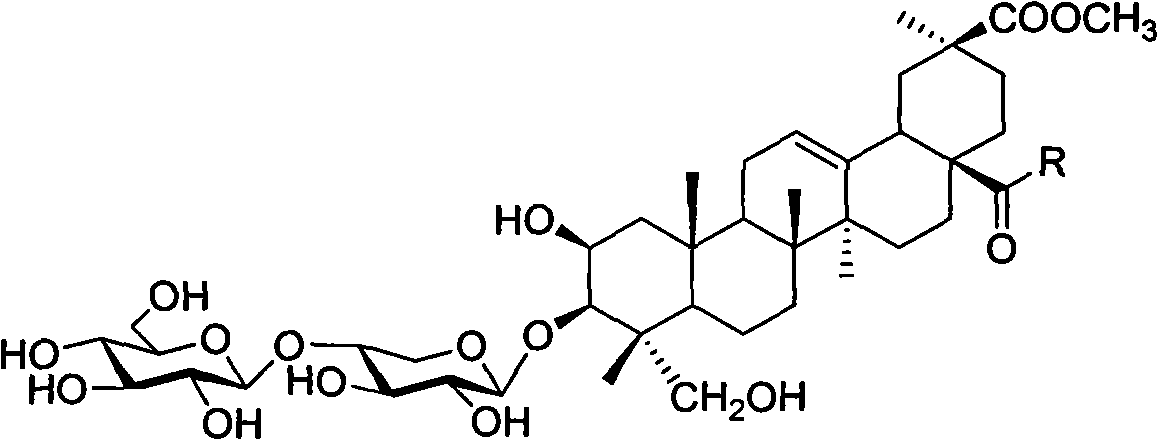
Esculentoside A, EsA as well as preparation method and application thereof
A technology of pokeweed saponin A and derivatives, which is applied in the field of medicine and can solve problems such as restricting the use of injection drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 3-O-[β-D-glucopyranose-(1,4)-β-D-xylopyranose]-2β, 3β, 23-trihydroxy-oleanane-12-ene- Synthesis of 28-(tyrosine methyl ester amide)-30-acid methyl ester
[0040] Dissolve pokeweed saponin A (EsA) (41.2 mg, 0.05 mmol) in dry DMF / THF (1:3, v / v), and put into N, N-dicyclohexylcarboimide (DCC, 20.7 mg , 0.1mmol) and N-hydroxybenzotriazole (HOBt, 13.5mg, 0.1mmol), stirred at room temperature for 1 hour, then added tyrosine methyl ester (39.0mg, 0.2mmol), under the protection of argon, heated to 60°C, stirred for 4 hours, filtered, separated by silica gel column chromatography (dichloromethane / methanol, 6:1), purified by HPLC (condition: C18, YMC 250×10mm, 5 μm; mobile phase: methanol / water, 75 %) to obtain 48.2 mg of white powder, the yield was 96%.
[0041] 1 H-NMR (600MHz, C 5 D. 5 N): δ6.89~6.86(m, 2H), 6.75~6.67(m, 2H), 6.58(brs, 1H), 5.39(t, 1H, J=3.6Hz), 5.01(d, 1H, J= 7.8Hz), 4.94(d, 1H, J=7.2Hz), 4.83(s, 1H), 4.79(m, 1H), 4.74(dd, 1H, J=2.4, 12.0Hz), ...
Embodiment 2
[0044] Example 2 3-O-[β-D-glucopyranose-(1,4)-β-D-xylopyranose]-2β, 3β, 23-trihydroxy-oleanane-12-ene- Synthesis of 28-(bisglycyl dipeptide methyl ester amide)-30-acid methyl ester
[0045] EsA (41.3mg, 0.05mmol) was dissolved in dry DMF / THF (1:3, v / v), and N, N-dicyclohexylcarboimide (DCC, 20.7mg, 0.1mmol) and N -Hydroxybenzotriazole (HOBt, 13.5mg, 0.1mmol), stirred at room temperature for 1 hour, then added diglycine dipeptide methyl ester (14.6mg, 0.1mmol), heated to 60°C under the protection of argon, Stir for 4 hours, filter, separate by silica gel column chromatography (dichloromethane / methanol, 7:1), and purify with HPLC (conditions: C18, YMC 250×10mm, 5 μm; mobile phase: methanol / water, 75%), to obtain White powder 42.7 mg, yield 91%.
[0046] 1 H-NMR (600MHz, C 5 D. 5 N): δ8.20(brs, 1H), 8.17(brs, 1H), 5.39(t, 1H, J=3.6Hz), 5.03(d, 1H, J=7.8Hz), 4.94(d, 1H, J =7.2Hz), 4.83(s, 1H), 4.79(m, 1H), 4.74(dd, 1H, J=2.4, 12.0Hz), 4.54~4.51(m, 1H), 4.33~4.28(m, 3H) , 4....
Embodiment 3
[0048] Example 3 3-O-[β-D-glucopyranose-(1,4)-β-D-xylopyranose]-2β,3β,23-trihydroxy-oleanane-12-ene- Synthesis of 28-[(3-amino)-1-propionamide]-30-acid methyl ester
[0049] EsA (1239 mg, 1.5 mmol) was dissolved in dry DMF / THF (1:3, v / v), and N, N-dicyclohexylcarboimide (DCC, 413 mg, 2 mmol) and N-hydroxybenzene And triazole (HOBt, 270mg, 2mmol), stirred at room temperature for 1 hour, then added 1,3-propanediamine (0.5ml, 6mmol), heated to 60°C under argon protection, stirred for 4 hours, filtered, Silica gel column chromatography (dichloromethane / methanol, 4:1), purified by HPLC (conditions: C18, YMC 250 × 10mm, 5 μm; mobile phase: methanol / water, 70%), to give white powder 1257mg, yield 95%.
[0050] 1 H-NMR (600MHz, C 5 D. 5N): δ6.50(brs, 1H), 5.39(t, 1H, J=3.6Hz), 5.01(d, 1H, J=7.8Hz), 4.96(d, 1H, J=7.2Hz), 4.79( d, 1H, J=1.2Hz), 4.67(dd, 1H, J=2.4, 12.0Hz), 4.33~4.28(m, 3H), 4.24~4.20(m, 2H), 4.18(d, 1H, J= 9Hz), 4.15(t, 1H, J=9Hz), 4.08(t, 1H, J=8.4Hz), 4.03(t, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com