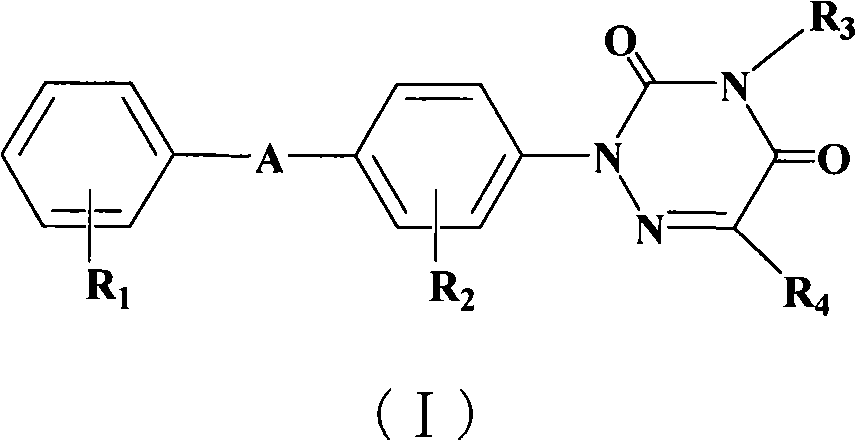
Triazine compounds having coccidiostat activity and preparation thereof
A compound, anti-coccidial technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, anti-infective drugs, etc., can solve the problems of antibiotic residues, excessive chicken and egg residues, toxicity hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Synthesis of 2-[4-(4'-trifluoromethylphenoxy)phenyl]-[1,2,4]-triazine-3,5-(2H,4H)-dione
[0062] Add 22.0g of p-hydroxyaniline, 25ml of water and 100ml of concentrated hydrochloric acid into the reaction flask, stir, cool down, add dropwise an aqueous solution containing 30.0g of sodium nitrite at 15~20°C, drop it in 30 minutes, keep it warm for 2 hours, and obtain the diazonium salt ,stand-by. Add 60.0g of ethyl malonyl dicarbamate, 300ml of pyridine, 300g of crushed ice and 200ml of water into another reaction flask. After cooling to below 10°C, pour the above diazonium salt slowly under vigorous stirring. After addition, react at 10~15°C for 5h. Filter and wash with water until neutral to obtain 70.3 g of ethyl 2-(4-hydroxyphenylhydrazone)-malonyl dicarbamate, with a yield of 95.1%.
[0063] Add 33.2g of 2-(4-hydroxyphenylhydrazone)-malonyldicarbamate, 20g of anhydrous sodium acetate and 250ml of glacial acetic acid into the reaction flask, heat and reflux for 5 ho...
Embodiment 2
[0068] 2-[4-(4'-trifluoromethylphenoxy)phenyl]-4-methyl-[1,2,4]-triazine-3,5-(2H,4H)-dione synthesis
[0069] 0.73g 2-[4-(4'-trifluoromethylphenoxy)phenyl]-[1,2,4]-triazine-3,5-(2H,4H)-dione, 0.1g Add sodium hydroxide and 20ml dimethyl sulfoxide into the reaction flask, stir at room temperature for 0.5h, add dropwise a mixed solution of 0.83g methyl iodide and 10ml dimethyl sulfoxide, and raise the temperature to 100°C after dropping, react for 5h, and the reaction is complete , down to room temperature, add about 100ml of water under stirring, filter, wash with water, and dry to obtain 0.67g of product with a yield of 88.5%.
Embodiment 3
[0071] Synthesis of 2-[4-(4'-nitrophenoxy)phenyl]-[1,2,4]-triazine-3,5-(2H,4H)-dione
[0072] Add 1.0 g of 2-(4-hydroxyphenyl)-[1,2,4]-triazine-3,5-(2H,4H)-dione, 0.75 g of anhydrous potassium carbonate and 40 ml of DMSO to the reaction flask , heated to 80°C and reacted for 30min with stirring, dissolved 0.8g p-nitrochlorobenzene in 10ml DMSO, added dropwise to the reaction flask, raised the temperature to 150°C and reacted for 10h, cooled down, added water (300ml), and adjusted the pH to 4 with dilute hydrochloric acid , filtered, washed with water, and dried to obtain 1.5 g of off-white powder product with a yield of 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com