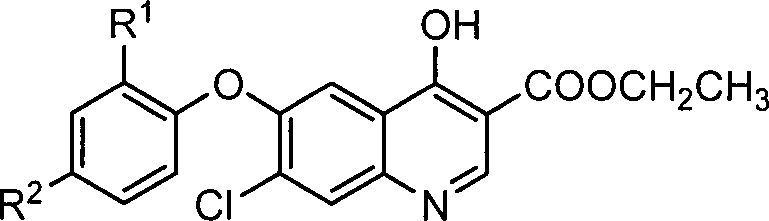
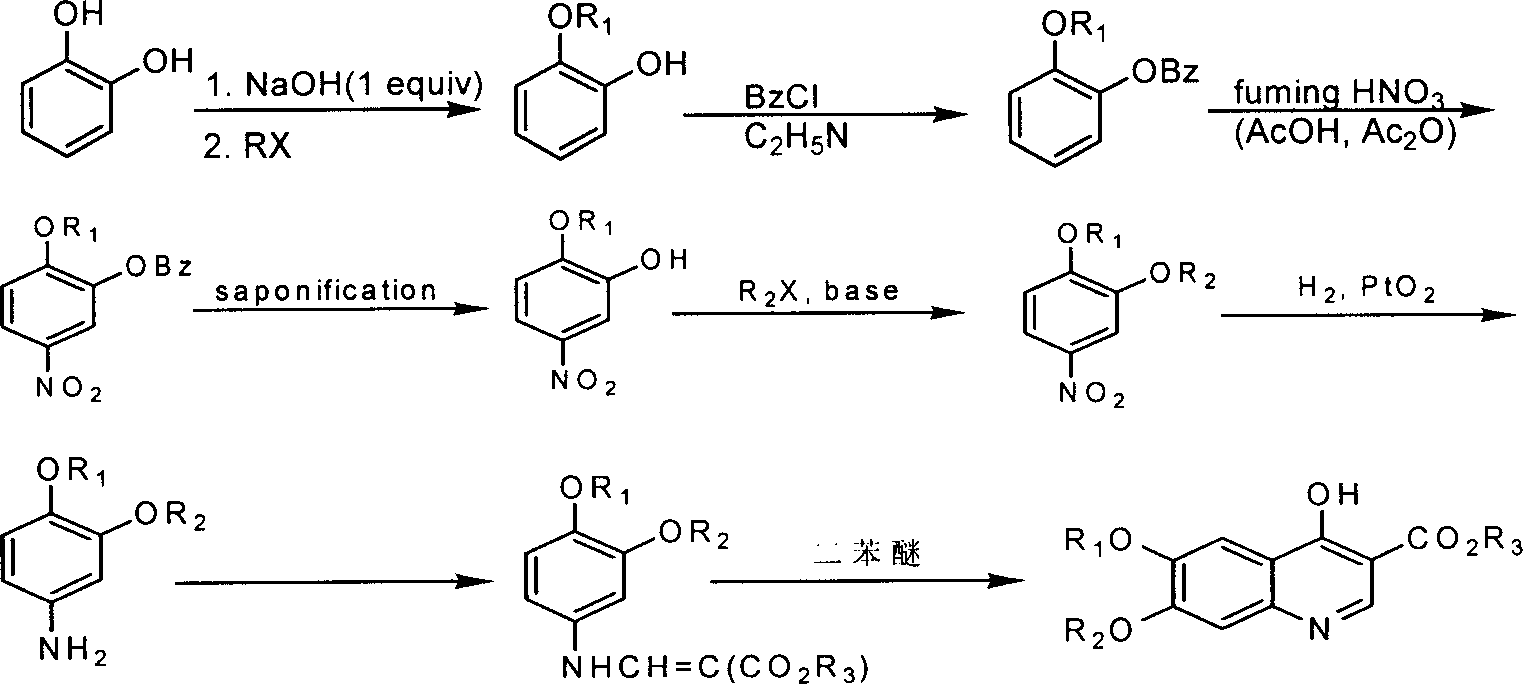
6-aryloxy-7-chloro-4-hydroxy-3-quinolinecarboxylate synthesis and uses
A technology of ethyl quinoline carboxylate and aryloxy, which is applied in the field of synthesis of ethyl 6-aryloxy-7-chloro-4-hydroxy-3-quinoline carboxylate, can solve the problem of high compound price and influence Problems such as mass use of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of target object 4a
[0025] (1) First add 13.66g (0.122mol) of p-fluorophenol in a 250ml three-necked bottle, heat and melt, then add 6.98g (0.122mol) of KOH, stir until all the generated water runs away, then add 19.3g ( 0.1 mol) of 1,2-dichloro-4-nitrobenzene, heated to an internal temperature of 150° C., reacted for 1 hour and 40 minutes, and detected the end point of the reaction by TLC. After the reaction, add 5% NaOH solution to the reaction system at 80°C to wash for 3 times, then wash with water twice at the same temperature, adjust the pH value to neutral with dilute HCl, pour the reaction solution into cold water while it is hot , cooled, suction filtered, and dried to obtain brown intermediate 1 with a yield of 84%.
[0026] (2) First add 22.50g (0.084mol) of intermediate 1 and 60ml of ethyl acetate into a 250ml round bottom flask, stir, and heat in a water bath until the external temperature is 40°C. After it is completely dissolv...
Embodiment 2
[0028] Embodiment 2: the preparation of target object 4b
[0029] Using p-bromophenol as raw material, the reaction steps are the same as above to obtain the target compound 4b.
Embodiment 3
[0030] Embodiment 3: the preparation of object 4c
[0031] Using p-methoxyphenol as raw material, the reaction steps are the same as above to obtain the target compound 4c.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com