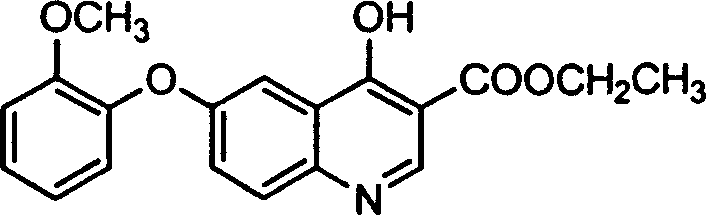
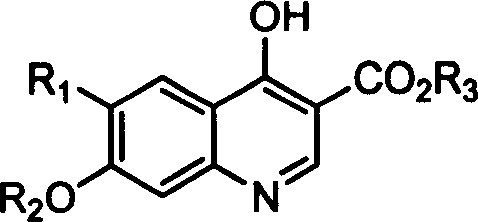
Synthesis and application of 6-(2-methoxy phenoxy)-1,4-dihydrogen-4-oxo-3-ethyl quinolinecarboxylate
A technology of ethyl quinolinecarboxylate and methoxyphenoxy, which is applied in the directions of active ingredients of heterocyclic compounds, drug combinations, anti-infective drugs, etc., can solve the problems of high compound prices and affecting the mass use of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of intermediate 1
[0027] (1) First add 16.31g (0.13mol) of o-methoxyphenol into a 250ml three-necked bottle, heat and melt, then add 7.37g (0.13mol) of KOH, stir until all the generated water runs away, then add 18.91 g (0.12mol) of p-chloronitrobenzene, then heated to 150°C, and TLC measured the end point of the reaction. After the reaction, add 5% NaOH solution to the reaction system at 100°C to wash for 3 times, then wash with water at the same temperature until the pH value is neutral, then pour into cold water to cool, filter, and dry to obtain a yellow intermediate 1 25.6 g, yield 87%.
[0028] (2) First add 16.31g (0.13mol) of o-methoxyphenol into a 250ml three-necked bottle, heat and melt, then add 7.37g (0.13mol) of KOH, stir until all the generated water runs away, then add 18.91 g (0.12mol) of p-chloronitrobenzene, and then heated to 120° C., and TLC detected the end point of the reaction. After the reaction, first add 5% Na...
Embodiment 2
[0029] Embodiment 2: the preparation of intermediate 2 and intermediate 3
[0030] (1) First add 21.78g (0.0888mol) of intermediate 1 and 100ml of ethyl acetate into a 250ml three-necked flask, and raise the external temperature to 40°C. After it is completely dissolved, add 5.5g of Pd / C, and then introduce H 2 It takes about 4.5 hours until the reaction system stops absorbing. After the reaction, filter, add 19.19g (0.0888mol) diethyl ethoxymethylenemalonate to the filtrate immediately, heat to reflux, and react for 4.5 hours. The end point of the reaction was detected by TLC. After the reaction, the ethyl acetate was distilled off under reduced pressure, and then recrystallized with absolute ethanol to obtain a light yellow solid, which was dried to obtain 27.78 g of yellow intermediate 3 with a yield of 78%.
[0031] (2) First add 21.78g (0.0888mol) of intermediate 1 and 100ml of ethyl acetate into a 250ml three-necked flask, and raise the external temperature to 20°C. Aft...
Embodiment 3
[0032] Embodiment 3: the preparation of target object
[0033] (1) First add 100ml of diphenyl ether into a 250ml three-necked bottle, and when the temperature is raised to 250°C, add 20g of intermediate 3 and react for 2h. After the reaction, cool down, add 100ml of petroleum ether, filter, and dry to obtain 15.5g of the brown-yellow target substance, with a yield of 92%.
[0034] (2) First add 100ml of diphenyl ether into a 250ml three-necked bottle, and when the temperature is raised to 220°C, add 20g of intermediate 3 and react for 4h. After the reaction, cool down, add 100ml of petroleum ether, filter, and dry to obtain 11.84g of the brown-yellow target substance, with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com