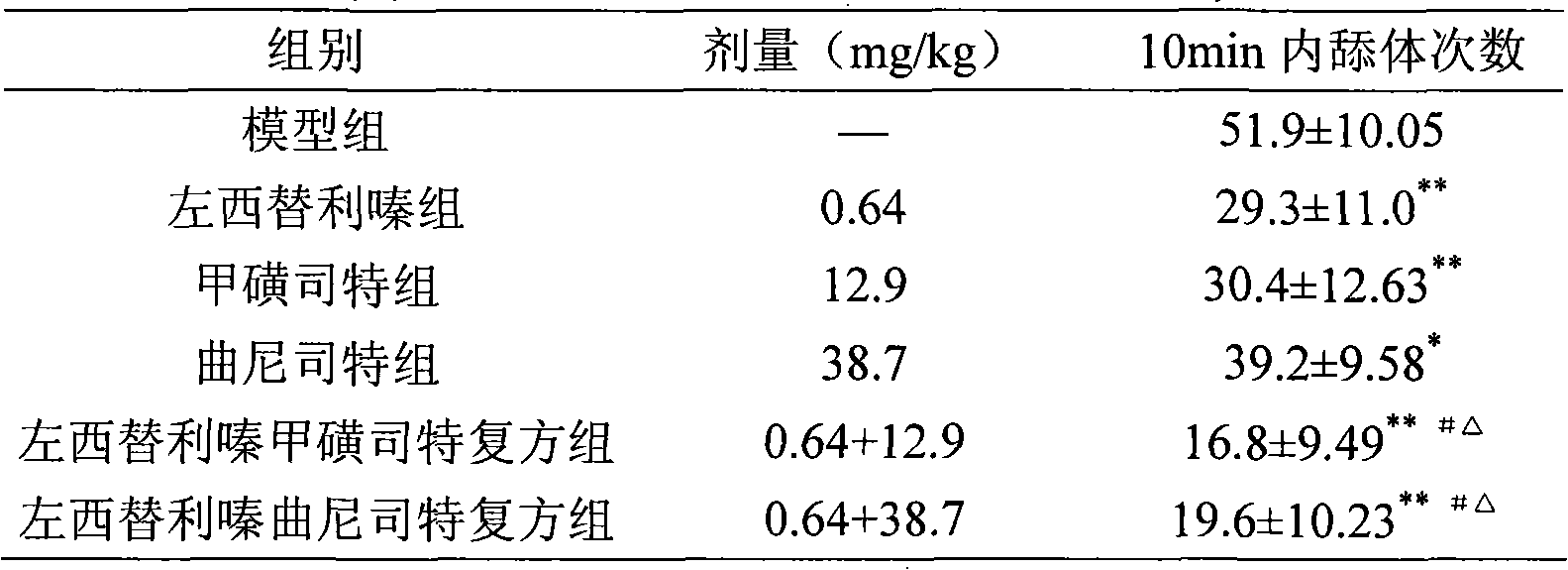
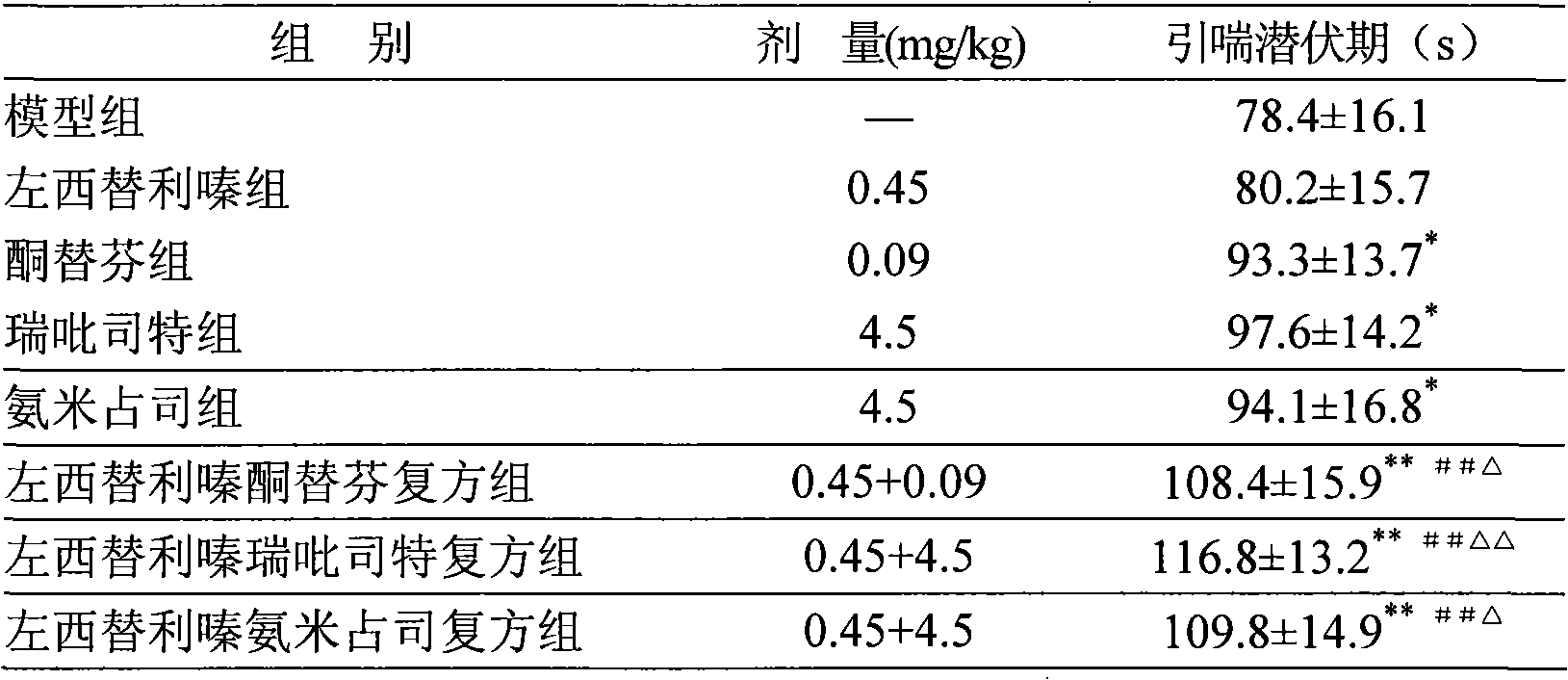
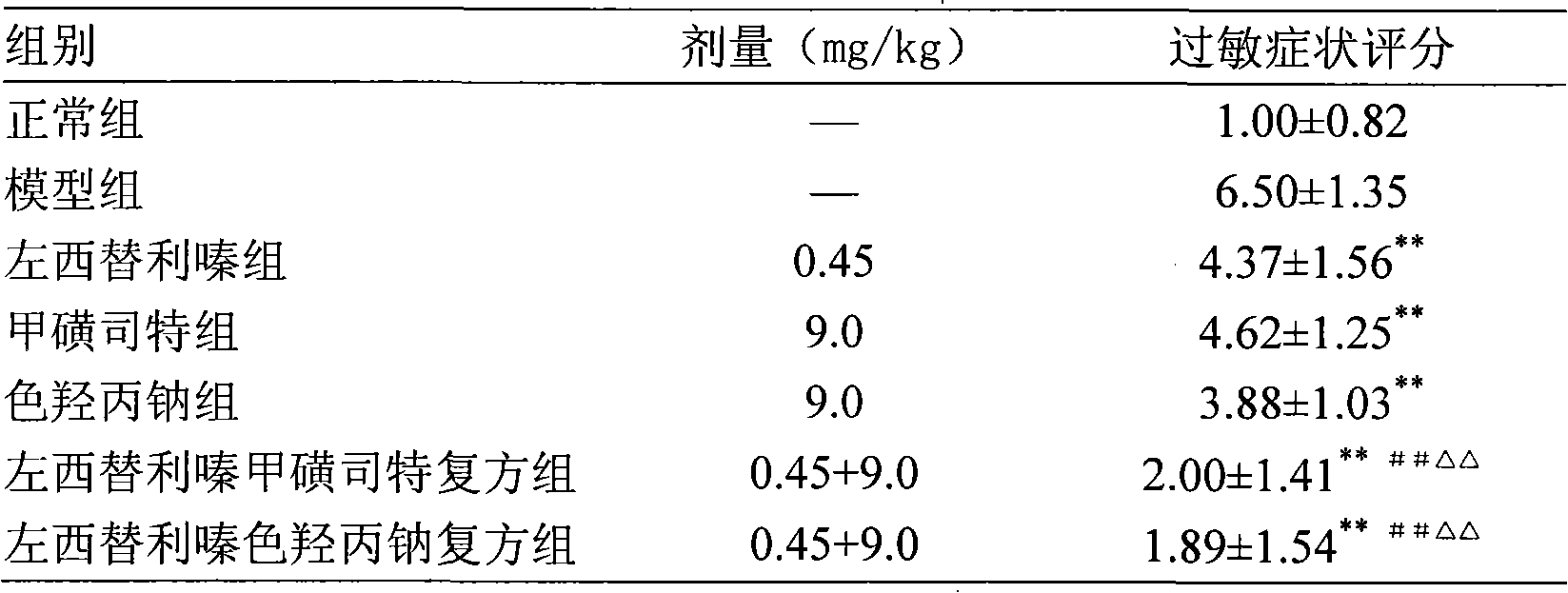
Oral anti-allergy compound pharmaceutical composition
A composition and anti-allergic technology, applied in the directions of drug combinations, allergic diseases, active ingredients of heterocyclic compounds, etc., can solve the problems such as levocetirizine and mast cell membrane stabilizers that have not yet been found, and achieve the superiority of anti-allergic reactions , prolong the incubation period of asthma, inhibit the effect of rhinitis allergic symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 Levocetirizine Ketotifen Tablets
[0017] formula:
[0018] Ingredient mg / composition
[0019] Levocetirizine 2.5
[0020] Starch 30.0
[0021] Lactose 70.0
[0022] Sodium carboxymethyl starch 5.0
[0023] 5% PVP K30 Solution Appropriate amount
[0024] Micronized silica gel 0.2
[0026] Ingredient mg / component
[0027] Ketotifen 0.5
[0028] Starch 10.0
[0029] Lactose 30.0
[0030] Sodium carboxymethyl starch 2.0
[0031] 5% PVP K30 Solution Appropriate amount
[0032] Micronized silica gel 0.1
[0034] Preparation method:
[0035] Take the formula amount of levocetirizine, starch, lactose, sodium carboxymethyl starch, mix evenly, put it in a high-speed stirring granulator, add an appropriate amount of binder, granulate, sieve, and dry at 50-60°C , add an appropriate amount of micropowder silica gel and magnesium stearate, and set aside; take the formulated amount of ketot...
Embodiment 2
[0036] Embodiment 2 Levocetirizine Ketotifen Capsules
[0037] formula:
[0038] Ingredient mg / composition
[0039] Levocetirizine 5.0
[0040] Starch 50.0
[0041] Lactose 80.0
[0042] Sodium carboxymethyl starch 6.5
[0043] 5% PVP K30 Solution Appropriate amount
[0044] Micronized silica gel 0.3
[0046] Ingredient mg / component
[0047] Ketotifen 1.0
[0048] Starch 15.0
[0049] Lactose 50.0
[0050] Sodium carboxymethyl starch 3.5
[0051] 5% PVP K30 Solution Appropriate amount
[0052] Micronized silica gel 0.2
[0053] Magnesium stearate 0.8
[0054] Preparation method:
[0055] Take the formula amount of levocetirizine, starch, lactose, sodium carboxymethyl starch, mix evenly, put it in a high-speed stirring granulator, add an appropriate amount of binder, granulate, sieve, and dry at 50-60°C , add an appropriate amount of micropowder silica gel and magnesium stearate, and set aside; take the formulated amount of keto...
Embodiment 3
[0056] Embodiment 3 levocetirizine tranilast capsules
[0057] formula:
[0058] Ingredient mg / composition
[0059] Levocetirizine 2.5
[0060] Tranilast 100
[0061] Starch 60.0
[0062] Lactose 90.0
[0063] Sodium carboxymethyl starch 6.0
[0064] 5% PVP K30 Solution Appropriate amount
[0065] Micronized silica gel 0.4
[0066] Magnesium stearate 1.5
[0067] Preparation method:
[0068] Get the levocetirizine, tranilast, starch, lactose, carboxymethyl starch sodium of formula quantity, mix, place high-speed stirring granulator, add appropriate amount of binder, granulate, sieve, in 50 Dry at ~60°C, add appropriate amount of micro-powdered silica gel and magnesium stearate, mix well and pack into capsules to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com