Naringin and levocetirizine hydrochloride pharmaceutical composition and preparation thereof
A technology of levocetirizine hydrochloride and its composition, which is applied in the field of naringin pharmaceutical composition and its preparation, can solve problems such as adverse reactions and death, and achieve good effects of relieving cough, reducing phlegm and calming asthma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
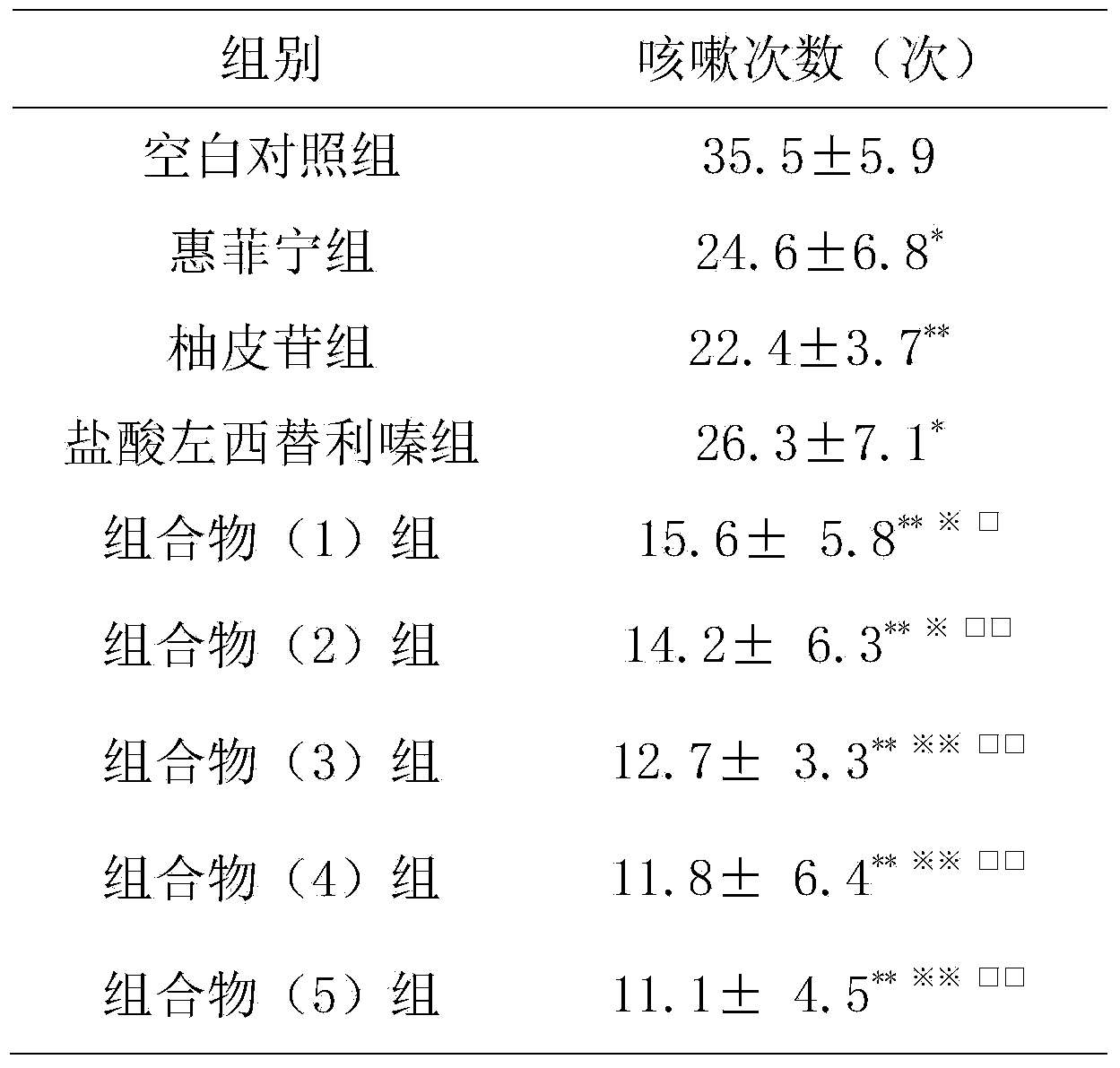
[0016] Inhibition of cough in guinea pigs induced by citric acid
[0017] 1. Materials
[0018] 1.1 Experimental animals Qualified Hartley guinea pigs, weighing 250-300g, male and female, were provided by the Guangdong Medical Experimental Animal Center.
[0019] 1.2 Drugs and reagents Huifei Ning; naringin is prepared according to the daily dosage of 120 mg; levocetirizine hydrochloride is prepared according to the daily dosage of 6 mg; the composition (1) group is prepared according to the daily dosage of 27.5 mg of naringin and levocetirizine hydrochloride Tirizine 1.25mg preparation; composition (2) group is prepared according to people's daily dosage of naringin 27.5mg, levocetirizine hydrochloride 12.5mg; composition (3) group is according to people's daily dosage of naringin 275mg, Cetirizine 1.25mg preparation; Composition (4) group is prepared according to human daily dosage of naringin 275mg, levocetirizine hydrochloride 12.5mg; Composition (5) group is prepared acc...
Embodiment 2
[0032] Effects on the Excretion Experiment of Phenol Red in Mice
[0033] 1. Materials
[0034] 1.1 Experimental animals Kunming mice, half male and half male, weighing 30-40 g, were provided by the Guangdong Medical Experimental Animal Center.
[0035] 1.2 Drugs and reagents: Ambroxol; naringin is prepared according to the daily dosage of 120 mg; levocetirizine hydrochloride is prepared according to the daily dosage of 6 mg; the composition (1) group is prepared according to the daily dosage of 27.5 mg of naringin and levocetirizine hydrochloride. Tirizine 1.25mg preparation; composition (2) group is prepared according to people's daily dosage of naringin 27.5mg, levocetirizine hydrochloride 12.5mg; composition (3) group is according to people's daily dosage of naringin 275mg, Cetirizine 1.25mg preparation; Composition (4) group is prepared according to human daily dosage of naringin 275mg, levocetirizine hydrochloride 12.5mg; Composition (5) group is prepared according to h...
Embodiment 3
[0048] Effect on ovalbumin-induced allergic cough (cough variant asthma)
[0049] 1. Materials
[0050] 1.1 Experimental animals: Hartley guinea pig, male, weighing 250-300 g, SPF grade, provided by Guangdong Medical Experimental Animal Center.
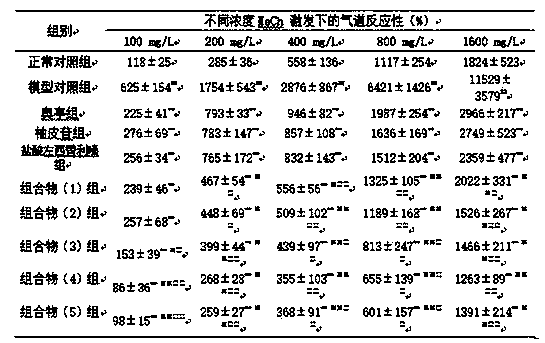
[0051] 1.2 Drugs and reagents Cyclophosphamide; Ovalbumin; Capsaicin; Methacholine; Aoting cough syrup (compound codeine phosphate solution); The daily dose of human is 6 mg; the composition (1) group is prepared according to the daily dose of naringin 27.5 mg and levocetirizine hydrochloride 1.25 mg; the composition (2) group is prepared according to the daily dose of naringin 27.5 mg and levocetirizine hydrochloride Cetirizine 12.5mg preparation; Composition (3) group is prepared according to the daily dosage of naringin 275mg, levocetirizine hydrochloride 1.25mg; Composition (4) group is prepared according to the daily dosage of naringin 275mg, 12.5 mg of cetirizine was prepared; the composition (5) group was prepared according t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com