New procedure for preparation of levocetirizine and its intermediates
A kind of technology of levocetirizine and intermediates, applied in the field of preparing levocetirizine and intermediates thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
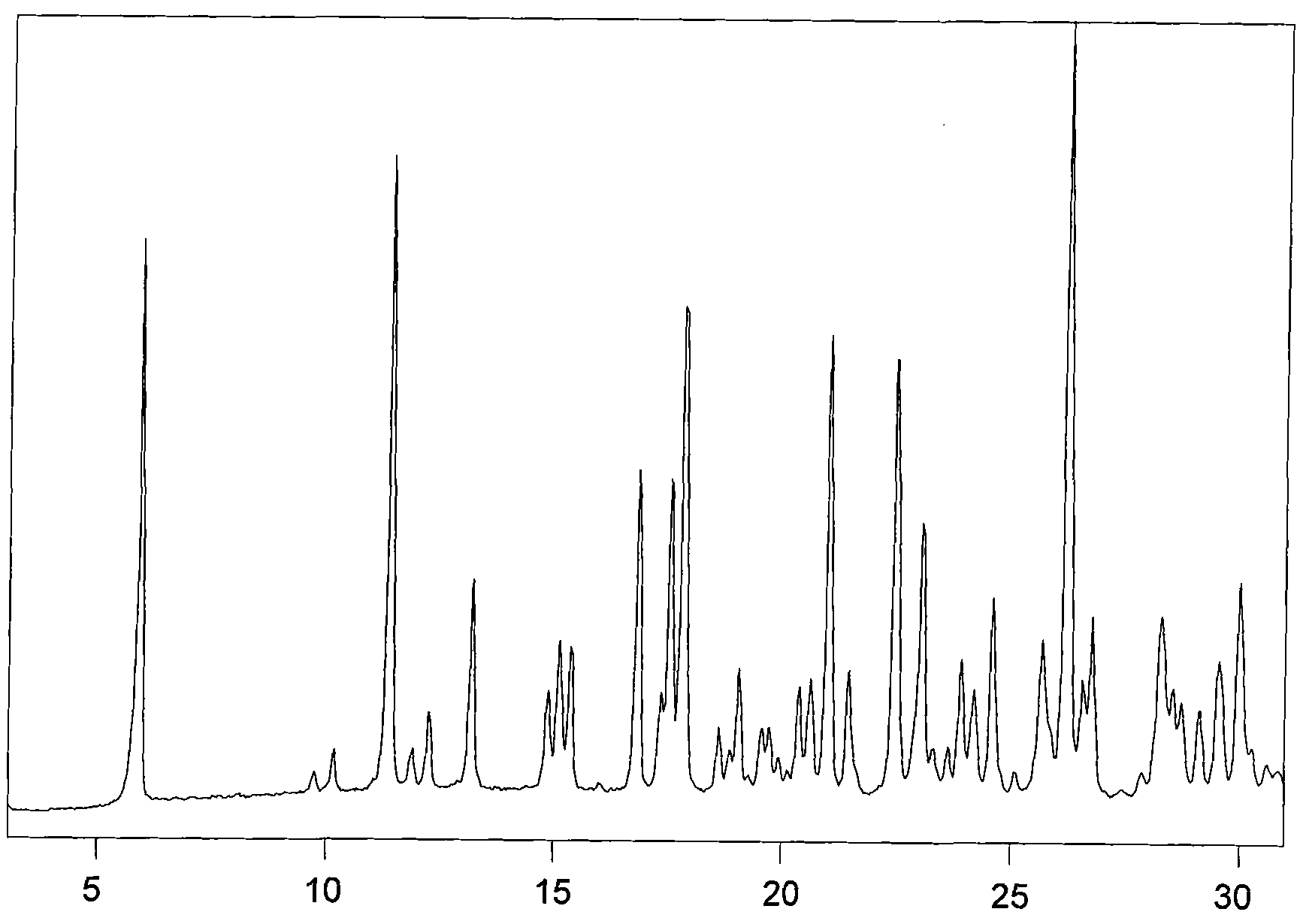
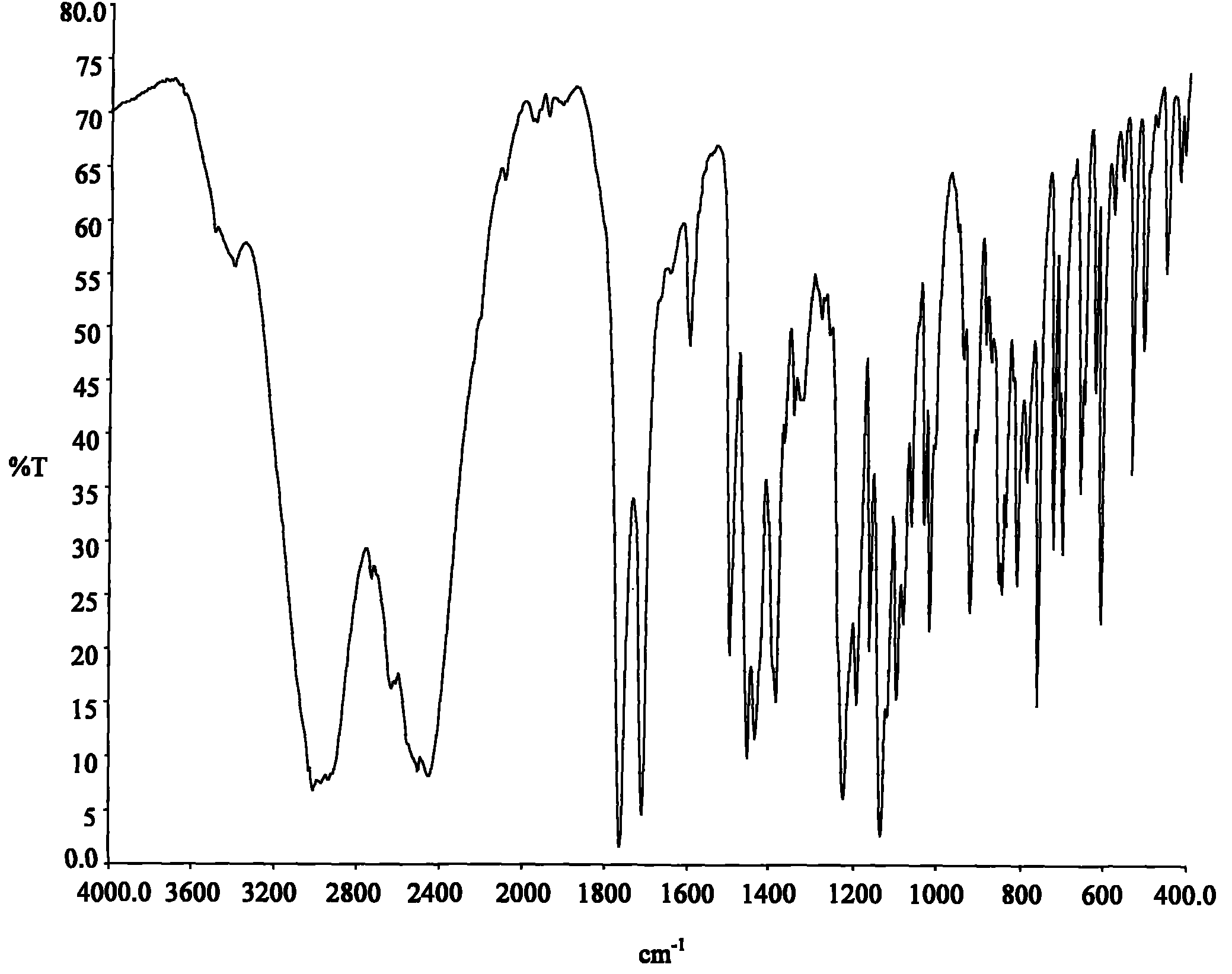
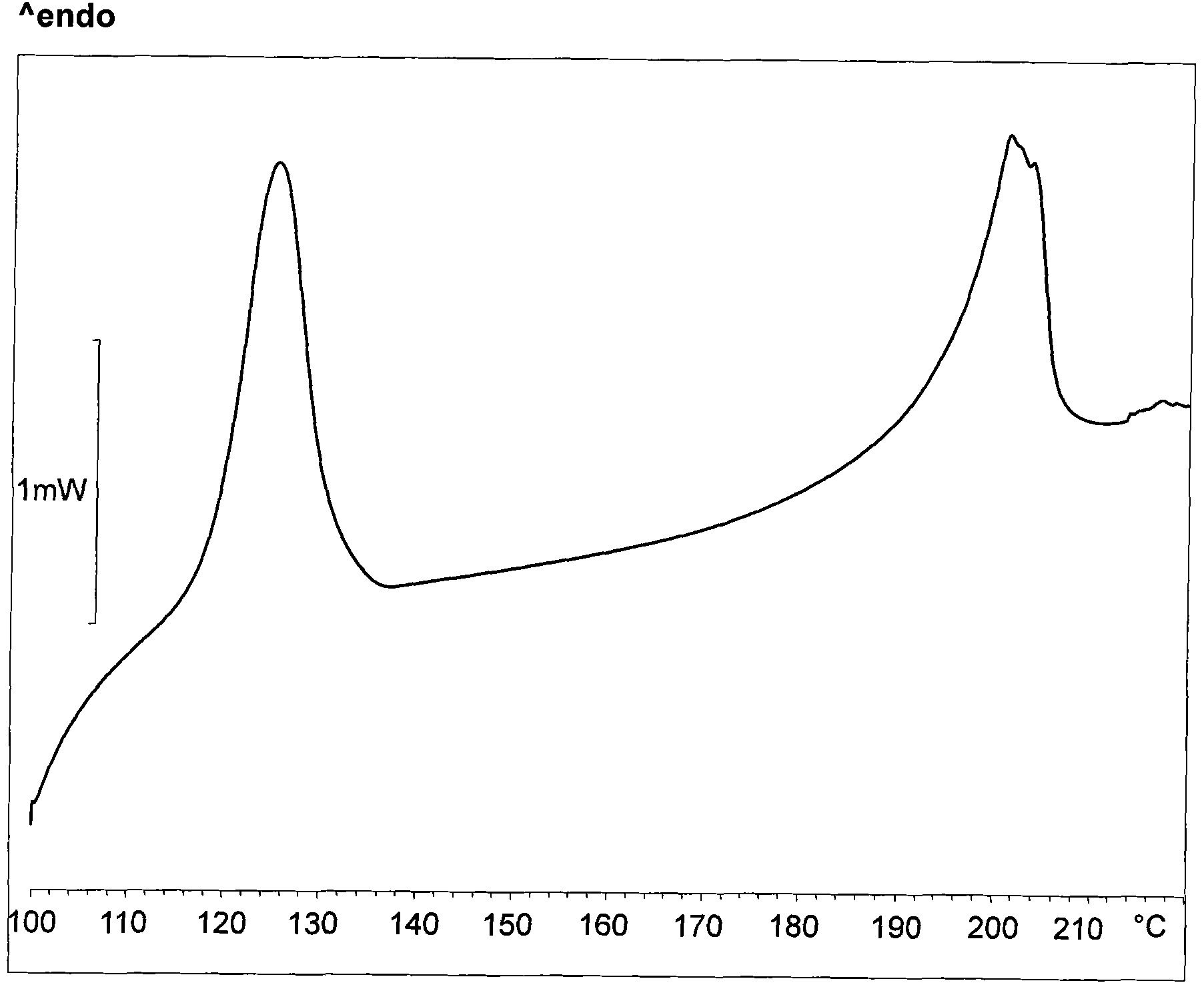
Image
Examples
Embodiment 1
[0104] (-)-1-[(4-Chlorophenyl)benzyl]-4-piperazine
[0105] Get 300mL of N-ethyldiisopropylamine, 30g of -(-)-4-chlorobenzhydrylamine (chlorobenzhydryamine) (Clemo, J.Chem.Soc.(1939) 1958-1969; Ingold, J .Chem.Soc.(1933)1493-1505), and 42g of two (2-chloroethyl) amine hydrochloride into the reaction vessel, stirring and heating and reflux for 3 hours. Cool to 60° C., add 24 mL of diethylamine, and then heat the mixture to reflux temperature for 5 hours. After the reaction was complete, the solvent was evaporated in vacuo, a mixture of water and ethyl acetate (1:1) was added, and the pH of the aqueous phase was adjusted to 10-11 with 30% sodium hydroxide solution. The organic phase was separated with ethyl acetate and the aqueous phase was extracted. The combined organic phases were washed with purified water, decolorized with charcoal and the filtrate was evaporated in vacuo. Impurities were separated using silica gel chromatography by eluting with ethyl acetate / ethanol (...
Embodiment 2
[0107] a) [2-[4-[(4-Chlorophenyl)benzyl]-1-piperazinyl]ethoxy]-acetonitrile dihydrochloride
[0108] Get 2400mL of acetonitrile into the reaction vessel, under stirring, add 400g of (-)-1-[(4-chlorophenyl)benzyl]-4-piperazine, 300g of Na 2 CO 3 , 20 g of KI and 300 g of 2-(2-chloroethoxy)acetonitrile.
[0109] Stir and gradually raise the temperature to 110-115°C. The temperature was maintained for 20 hours, and after the reaction was complete, the mixture was cooled to 80-90° C., and 25 g of activated carbon was added and stirred for 20 minutes. The carbon was filtered off and the filter cake was washed with appropriate amount of acetonitrile. Dry HCl gas was introduced into the combined filtrates until the pH reached 0.5-1. Stirring of the slurry was continued for 20 minutes then filtered. The filter cake was washed with appropriate amount of ethanol and dried at 50-55°C for 10 hours to obtain 520 g of the title product.
[0110] Yield was 95%, HPLC (area) 95%. Obta...
Embodiment 3
[0114] Levocetirizine
[0115] The methyl alcohol of 3000mL and the [2-[4-[(4-chlorophenyl) benzyl]-1-piperazine] ethoxyl group]-acetonitrile dihydrochloride of 300g are added in the reaction vessel, keep stirring A KOH solution prepared by adding 600 g of KOH and 600 mL of purified water to it under the condition that the reaction temperature is lower than 40° C. The temperature was gradually increased to 70-76°C and maintained for 24 hours. After the reaction was completed, the reaction mixture was cooled to 40-45° C., and methanol was distilled off under reduced pressure. Then, add 1000 mL of purified water and 3000 mL of CH 2 Cl 2 , and lower the temperature to 20-30°C. Adjust the pH to 4.2-4.8 with 37% HCl. Separate the organic layer and wash with 2000 mL of CH 2 Cl 2 Extract the aqueous layer twice.
[0116] The organic phases were combined and dried over 200 g of anhydrous sodium sulfate for 1 hour and filtered. The organic phase was concentrated until an oil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com