Levocetirizine preparation method and levocetirizine dihydrochloride preparation method
A technology of levocetirizine and levocetirizine, applied in the field of medicine and chemical industry, can solve the problems of environmental pollution, few reaction steps, expensive Pt/C catalyst, etc., and achieve the effect of high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
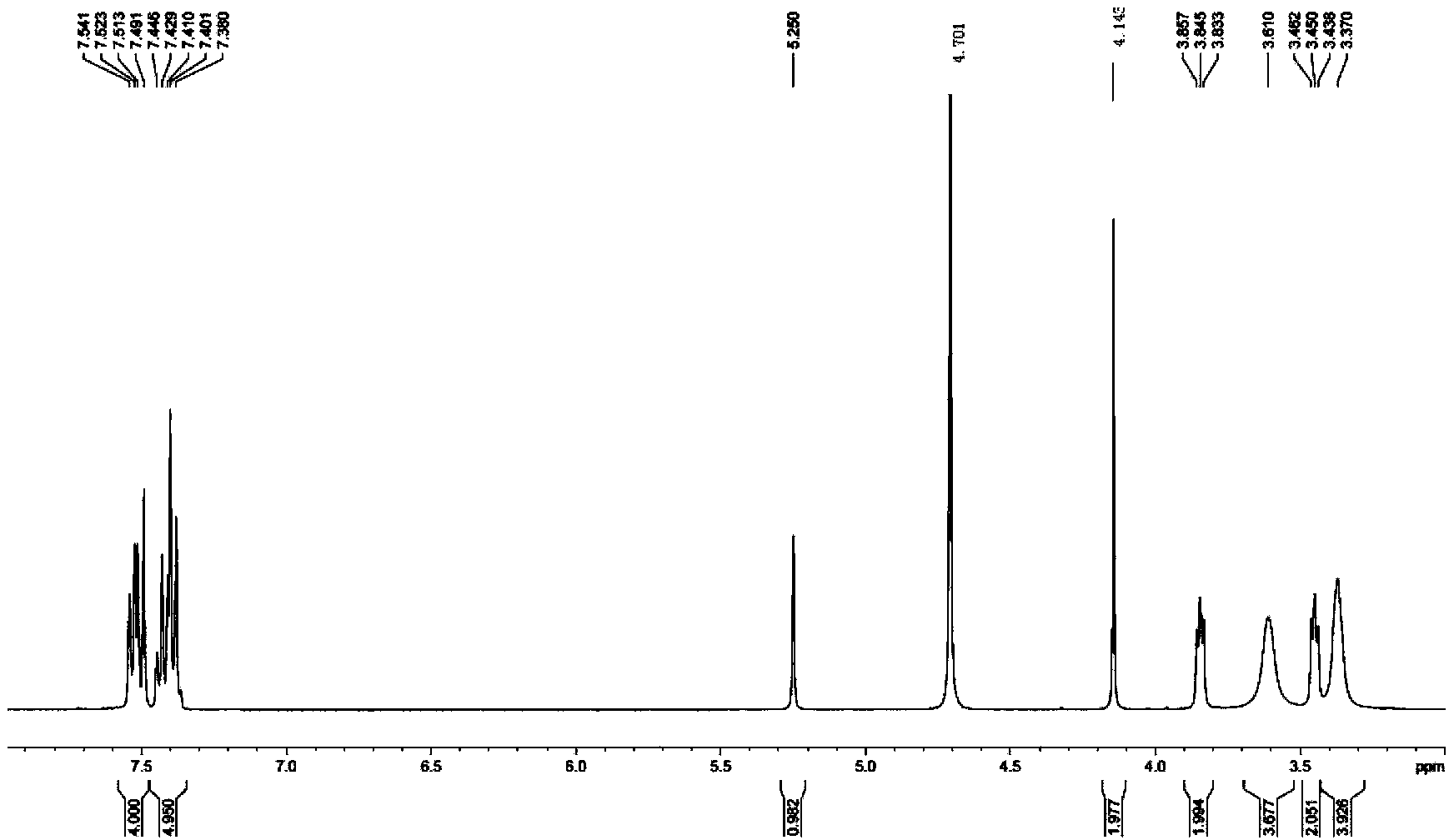
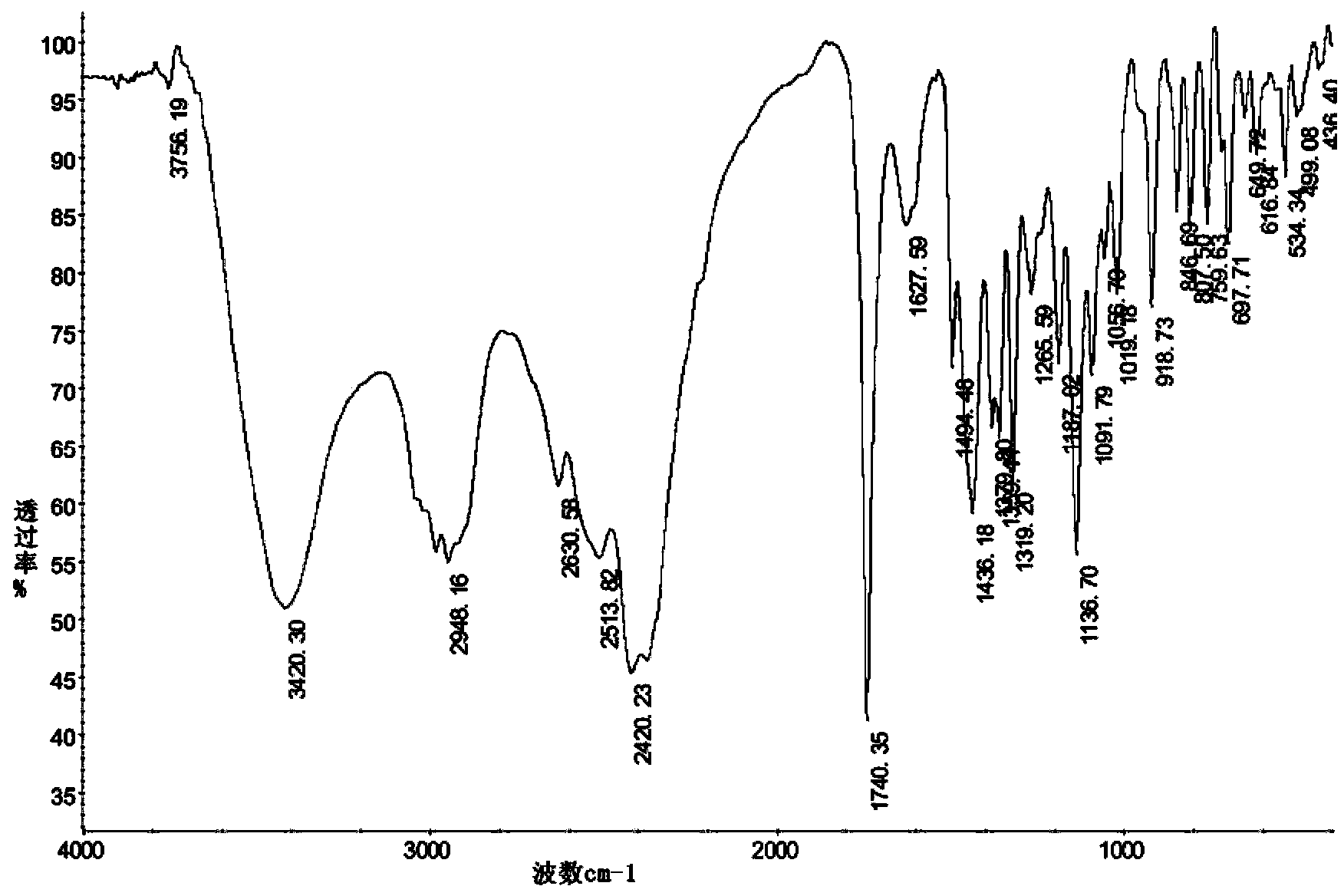
[0065] Synthesis of Levo-Hydroxyzine Dihydrochloride
[0066]
[0067] Add 2.9g (0.01mol) (R)-4-chlorobenzhydrylpiperazine to 100mL three-necked flask and dissolve in 10ml ethyl acetate, then add 3.0g K 2 CO 3 And 0.05g tetrabutylammonium bromide, stir well. Dissolve 2.0g (0.016mol) of monochlorodiethylene glycol in 10mL of water and add dropwise to the above reaction system. After the dropwise addition, raise the temperature to 80°C and reflux for 5h, track with TLC to determine the end point of the reaction (developer: methanol / chloroform =1:5 (v / v)), after the reaction is completed, cool to room temperature, transfer the reaction solution into a separatory funnel, separate the water layer, add toluene for extraction (10mL×2), and initially separate the extracted organic layer from the reaction solution to obtain The organic layers were combined, ethyl acetate was distilled off, and then toluene was added until completely dissolved, then washed with water (6×2mL) and wa...
Embodiment 2
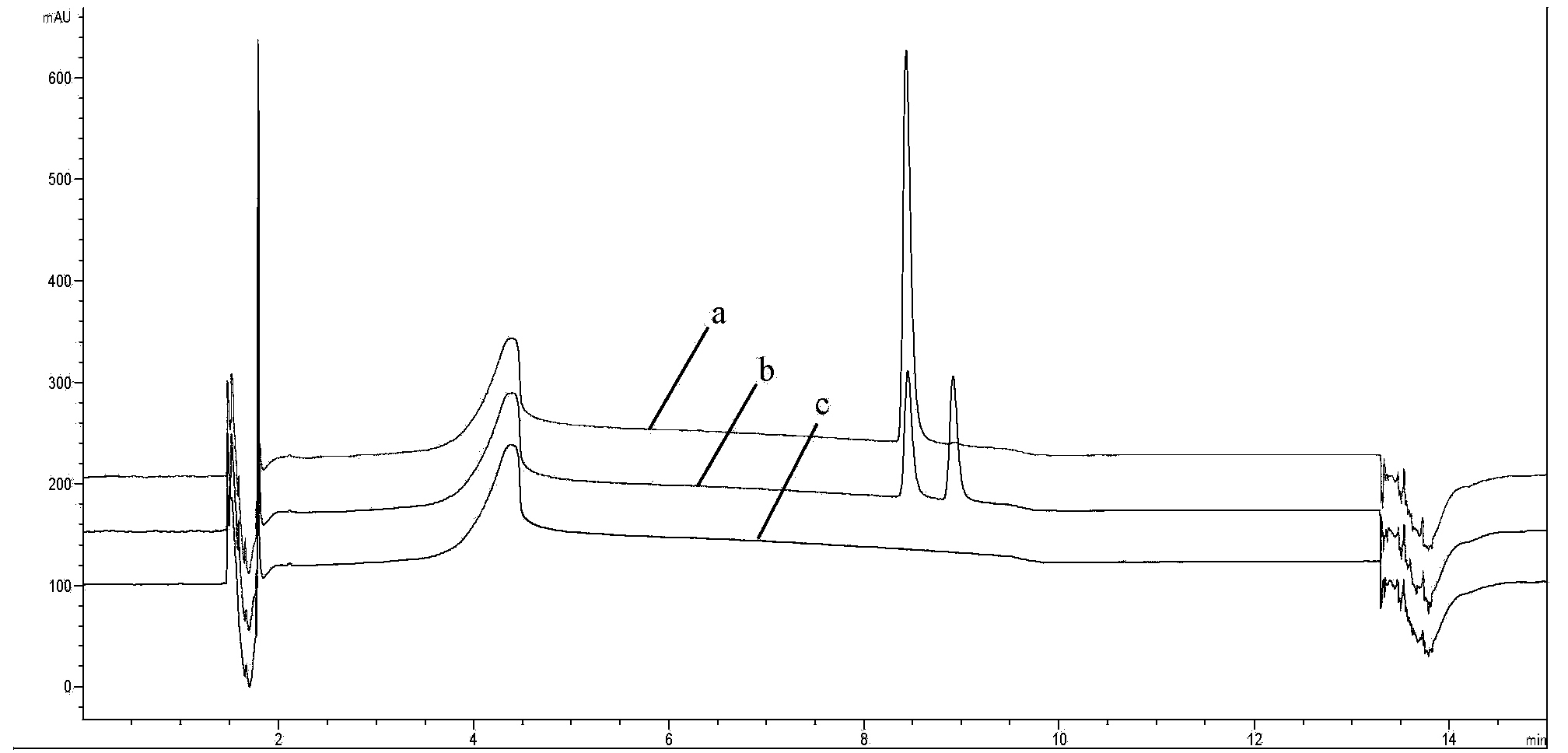
[0075] Add 0.01mol L-hydroxyzine to a 100ml reactor, add 50ml of a mixed solvent of 1.4-dioxane and distilled water (1:1, v / v) and stir to form a homogeneous phase, then add 2ml of 2mol / L NaOH solution to adjust the pH =12.5, finally add 0.75g catalyst to feed oxygen, heat to reflux, and in the reflux reaction process, the pH of the system is maintained at 12.5 by dripping 2mol / L of NaOH, TLC monitors the reaction until the left-handedness disappears completely, stops the reaction, and takes a sample to neutralize To pH~8, carry out HPLC analysis, the result is as follows:
[0076] Reaction ID catalyst Conversion rate 1 % selectivity% Chromatographic yield 2 % 1 5%Pd / C 95.1 95.3 91 2 1O%Pd / C 98.5 97.7 96.2 3 5%Pd / C-1%Pb / C 94.5 896 84.7 4 5%pd / C-3%Co / C 97.7 95.4 93.2 5 5%Pd / C-1%Bi / C 98.9 98.7 97.6
[0077] 1 Conversion %, based on consumption of raw material L-hydroxyzine
[0078] 2 Chromatographic yield %,...
Embodiment 3
[0080] In order to confirm the process stability, under the reaction conditions described in Example 2, the experiment was repeated three times with the catalyst numbered 5, and the chromatographic yields of the three times were respectively: 97.2%, 96.8%, and 97.6%.
[0081] The results show that the process has good stability and stable yield, and the average chromatographic yield is 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com