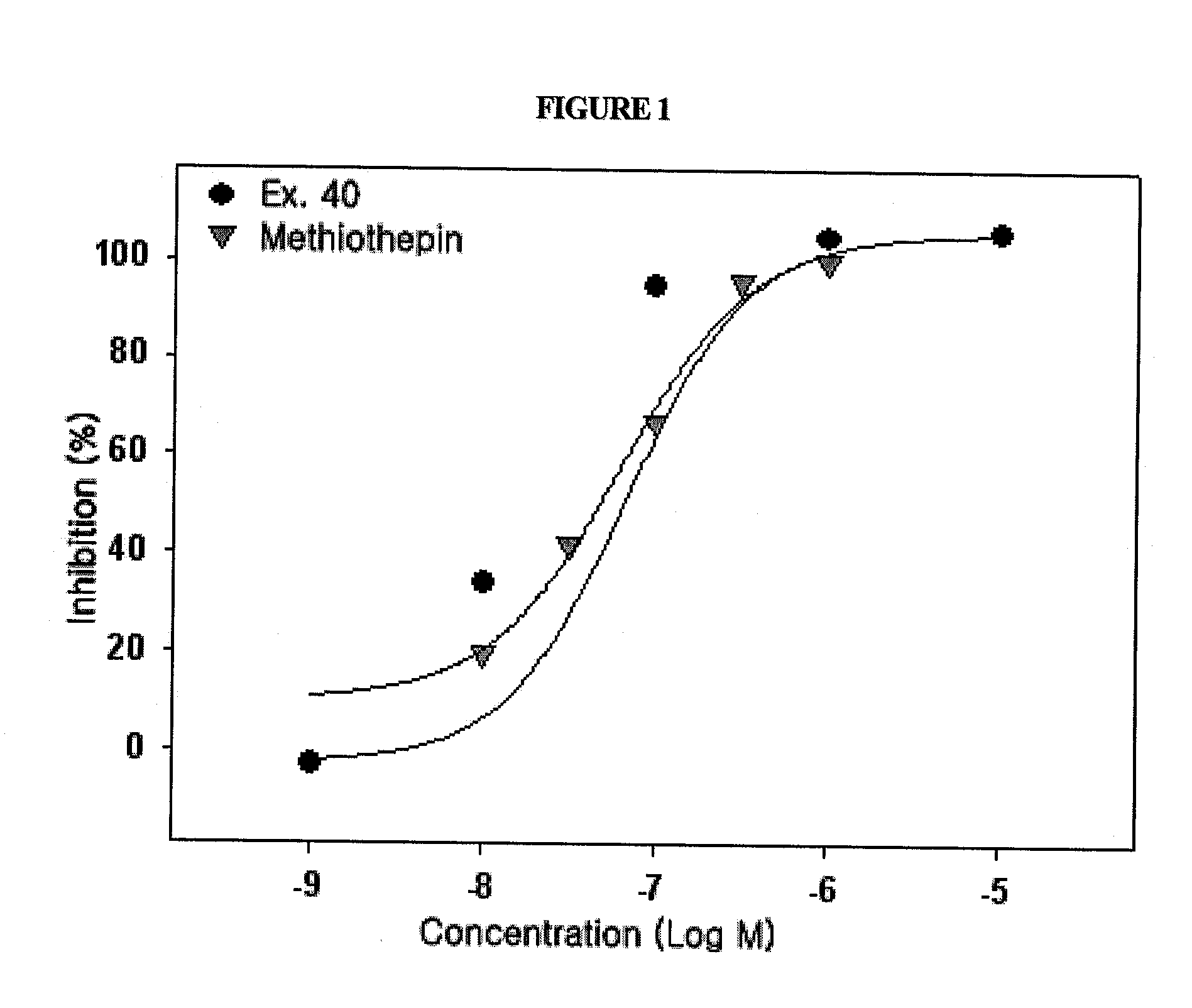
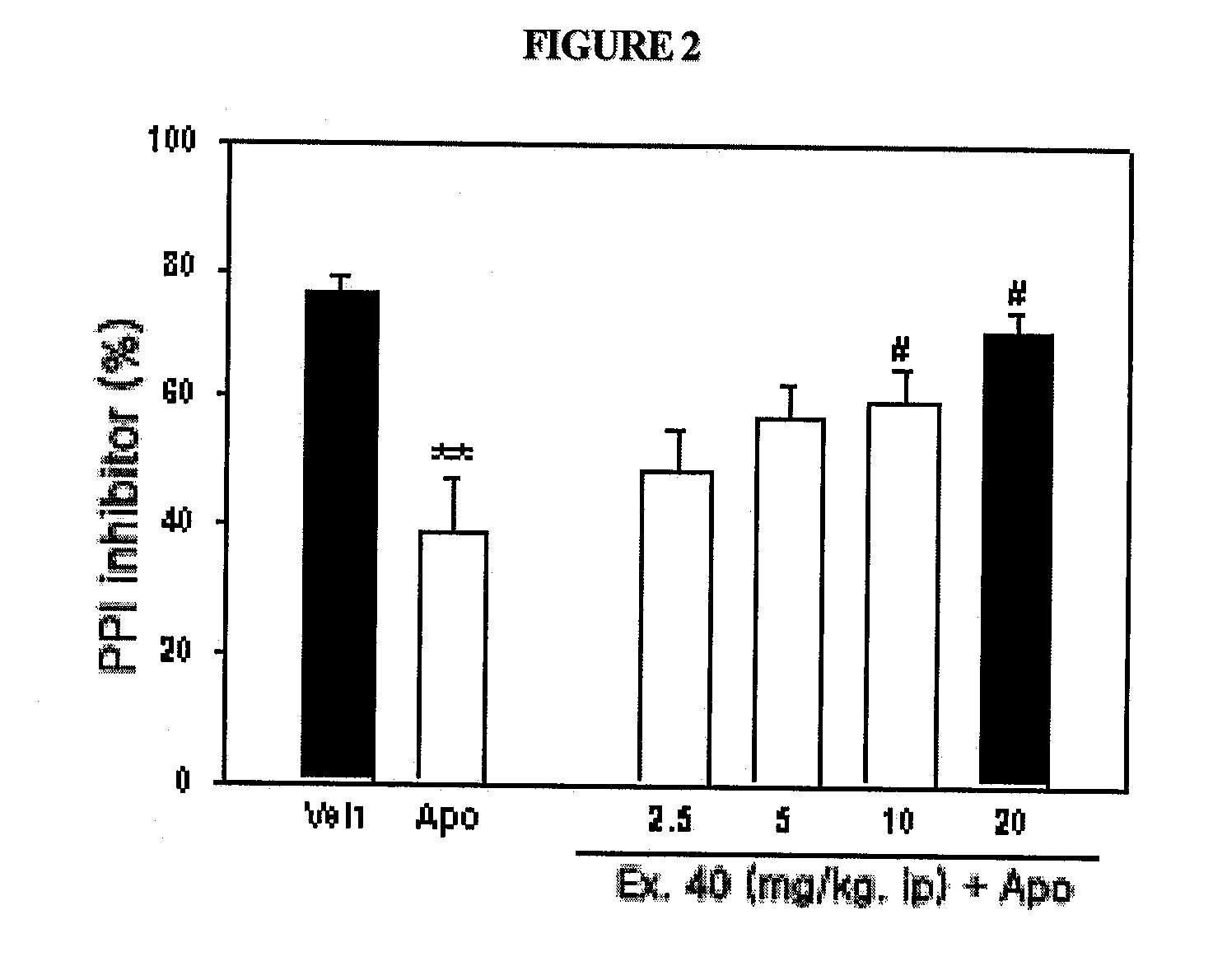
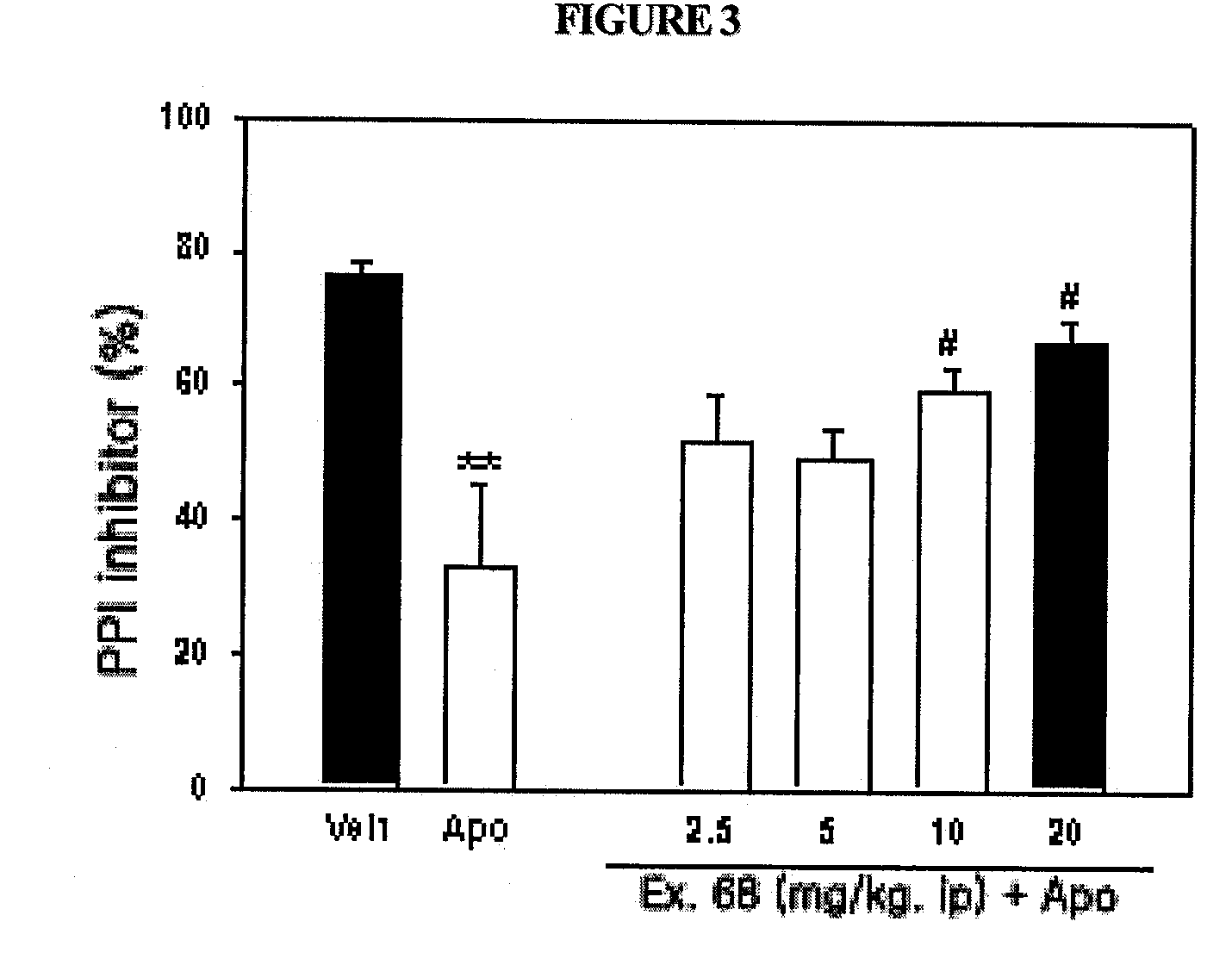
Novel substituted-1-h-quinazoline-2,4-dione derivatives, preparation method thereof and pharmaceutical composition containing the same
a technology of hquinazoline and derivatives, which is applied in the field of new substituted hquinazoline2, 4dione derivatives, can solve the problems of limited value in most other pharmacological studies, low bioavailability, and low penetration into the cns, and achieve excellent binding affinity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 2
Preparation of Intermediate I
preparation example 2-1
Preparation of 2-amino-4,6-dichloro-N-methyl-benzamide (Intermediate I-1)
[0170]A solution of 5,7-dichloroanthranilic anhydride (2.00 mmol), methylamine (2.40 mmol) and TEA (2.40 mmol) in tetrahydrofuran or 1,4-dioxane (30 ml) was heated for 2 hours with flux. After the starting material anhydride disappeared, the solvent was removed through vacuum drying. The residue was washed with 1 N hydrochloric acid (100 ml). Recrystallization from a solvent containing methanol, ethylacetate or benzene for corresponding N-substituted benzamide yielded the title compound as a bright brown solid (62%).
[0171]m.p. 159-162° C.;
[0172]1H NMR (200 MHz, CDCl3+CD3OD) δ 2.95 (d, 3H, J=4.9 Hz, NHCH3), 6.63 (d, 1H, J=2.0 Hz, ArH), 6.71 (d, 1H, J=2.0 Hz, ArH), 7.12 (br s, 1H, NH);
[0173]MS (EI) m / e 219 [M+], 188, 148, 133, 73.
preparation example 2-2
Preparation of 2-amino-4,6-dichloro-N-ethyl-benzamide (Intermediate I-2)
[0174]The same procedure as in Preparation Example 2-1, with the exception mat 5,7-dichloroanthranilic acid and ethylamine were used, was performed to give the title compound as a bright brown solid (64%).
[0175]1H NMR (200 MHz, CD3OD) δ 1.25 (t, 3H, J=7.3 Hz, CH3), 3.15 (q, 2H, J=7.3 Hz, NHCH2), 7.01 (d, 1H, J=2.0 Hz, ArH), 8.07 (d, 1H, J=2.0 Hz, ArH);
[0176]MS (EI) m / e 232 [M+], 205, 162, 72.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com