Preparing method of phosphodiesterase 5 inhibitor tadalafil
A technology of phosphodiesterase and tadalafil, applied in the direction of organic chemistry, to achieve the effect of mild conditions and less volatilization of methylamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
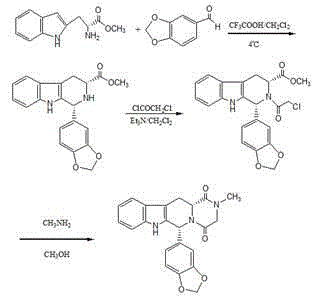
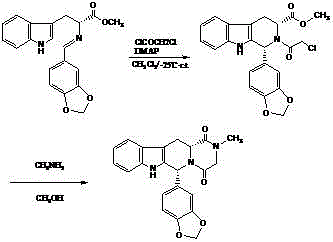
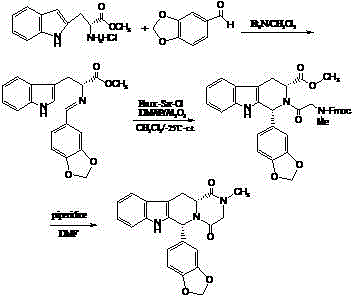
[0044] 1, 1R, 3R)-1,2,3,4-tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3- Preparation of methyl carboxylate hydrochloride (compound IV):
[0045] D-tryptophan methyl ester hydrochloride (100 g, 0.39 mol), 20 ml of isopropanol, 1000 ml of acetonitrile, piperonal (65 g, 0.43 mol) were mixed and stirred to reflux for 11 to 13 hours, cooled to room temperature, filtered, Washed with isopropanol and dried to obtain 144.0 g (94.9%) of compound IV with an ee value of 99.6%, [α] D 20 =+82.0 0 (c1, CH 3 OH).
[0046] 2. (1R, 3R)-1,2,3,4-tetrahydro-2-chloroacetyl-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4- b] Preparation of methyl indole-3-carboxylate (compound V):
[0047] Compound IV (48 g, 0.12 mol) was suspended in 1.0 liter of dichloromethane, added 50 ml of triethylamine, stirred for 30 minutes, cooled at 10°C, added dropwise with chloroacetyl chloride (14 ml, 0.18 mol), then stirred for 1.5 hours , 500 ml of 10wt% potassium carbonate solution was ad...
Embodiment 2
[0061] According to Example 1: in step 1, 1000 ml of isopropanol was used as the reaction solvent instead of the mixed solvent of isopropanol and acetonitrile to obtain compound IV with a yield of 89.5% and an ee value of 98.1%, [α] D 20 =+79.0 0 (c1, CH 3 OH).
Embodiment 3
[0063] According to Example 1, 1000 ml of acetonitrile was used as the reaction solvent in step 1 instead of the mixed solvent of isopropanol and acetonitrile to obtain compound IV with a yield of 91.0% and an ee value of 99.0%, [α] D 20 =+81.0 0 (c1, CH 3 OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com