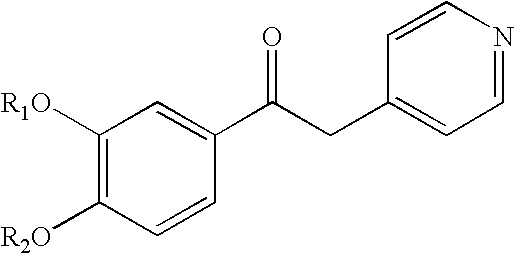
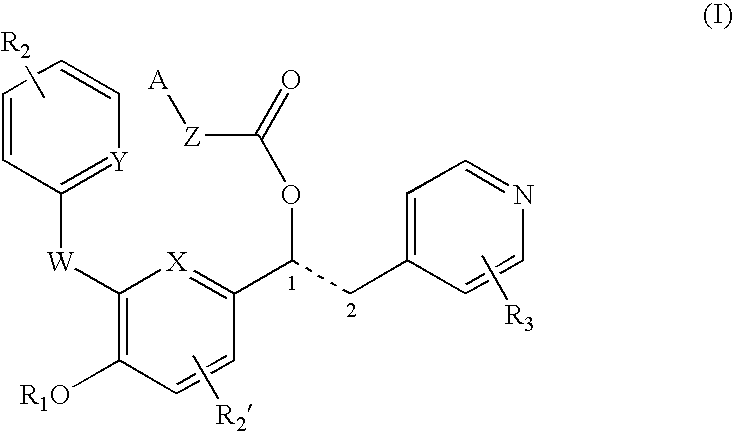
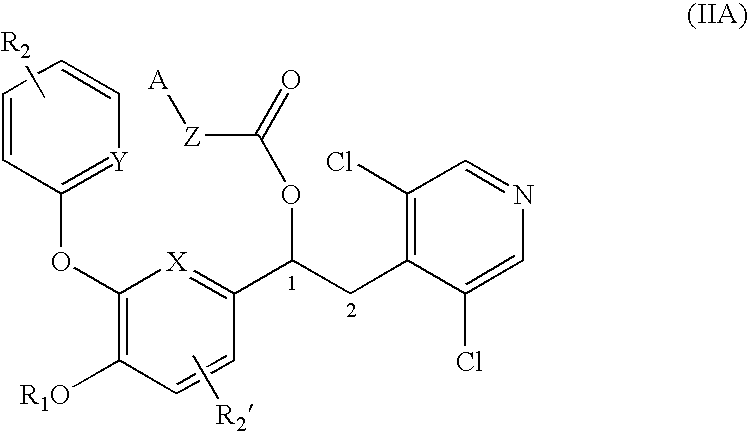
Patents
Literature
249 results about "Phosphodiester alpha" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
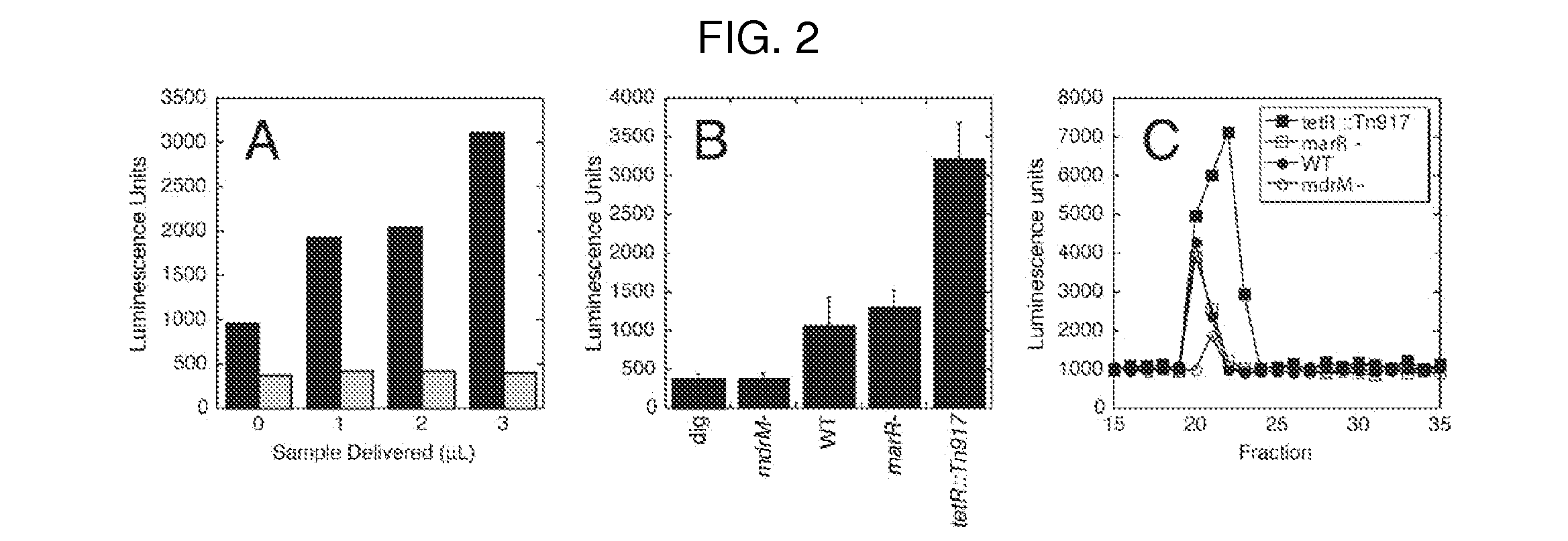
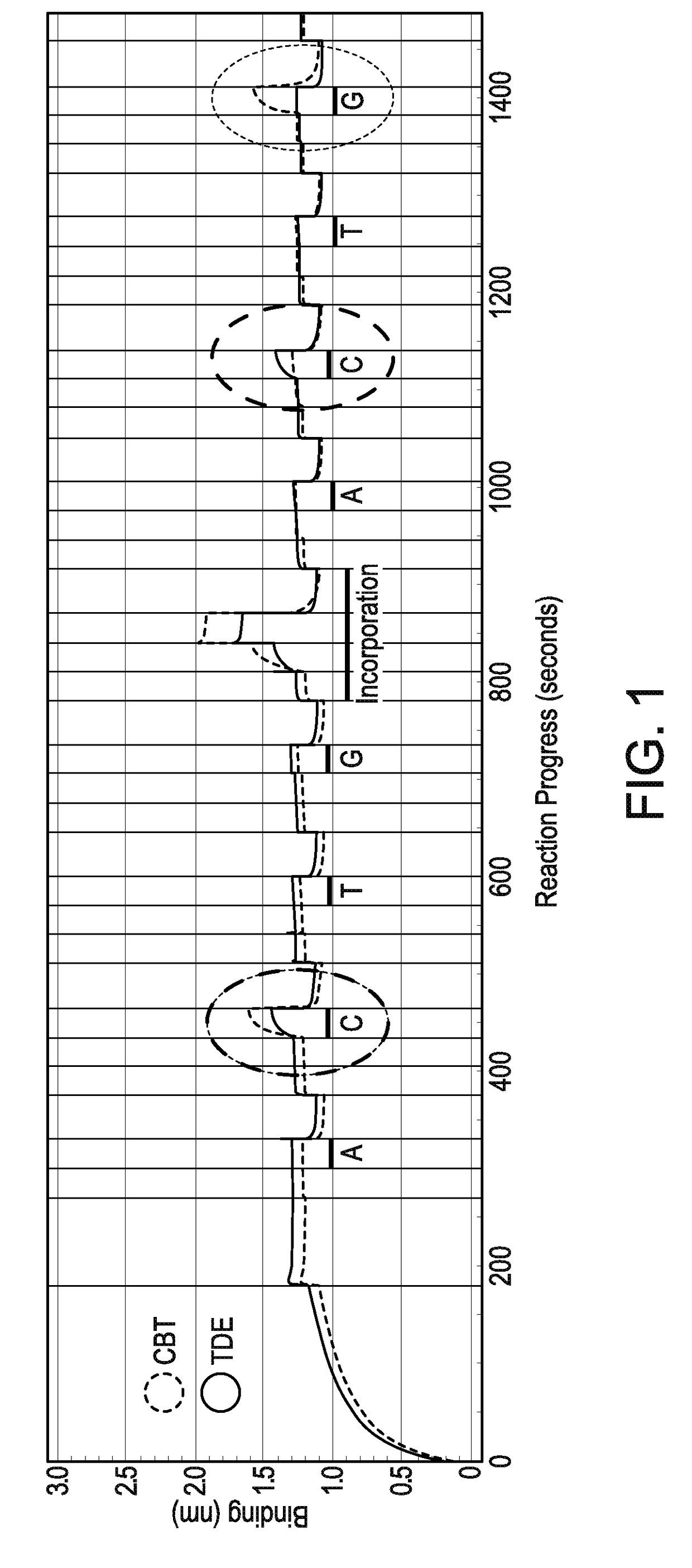
Methods and compositions for improving fidelity in a nucleic acid synthesis reaction
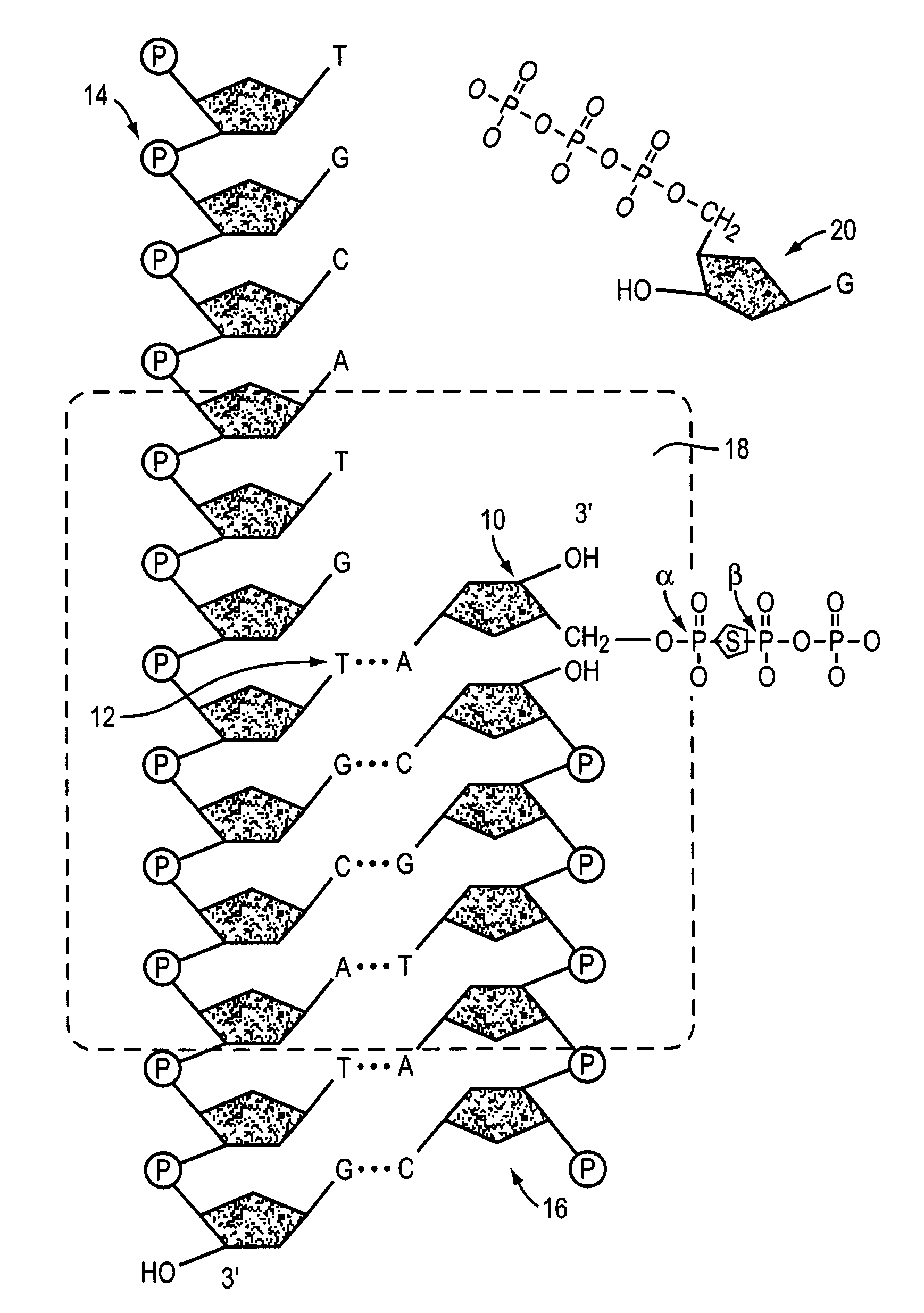
InactiveUS7482120B2Improve fidelityInhibition formationMicrobiological testing/measurementFermentationNucleotidePerylene derivatives
The invention provides methods and compositions for improving the fidelity of a sequencing-by-synthesis reaction by using a nucleotide derivative that forms a hydrogen bond with a complementary nucleotide on a template, but fails to form a phosphodiester bond with the 3′ hydroxyl group of a primer under conditions otherwise suitable for a polymerization reaction; thereby blocking incorporation of a mismatched nucleotide.
Owner:FLUIDIGM CORP
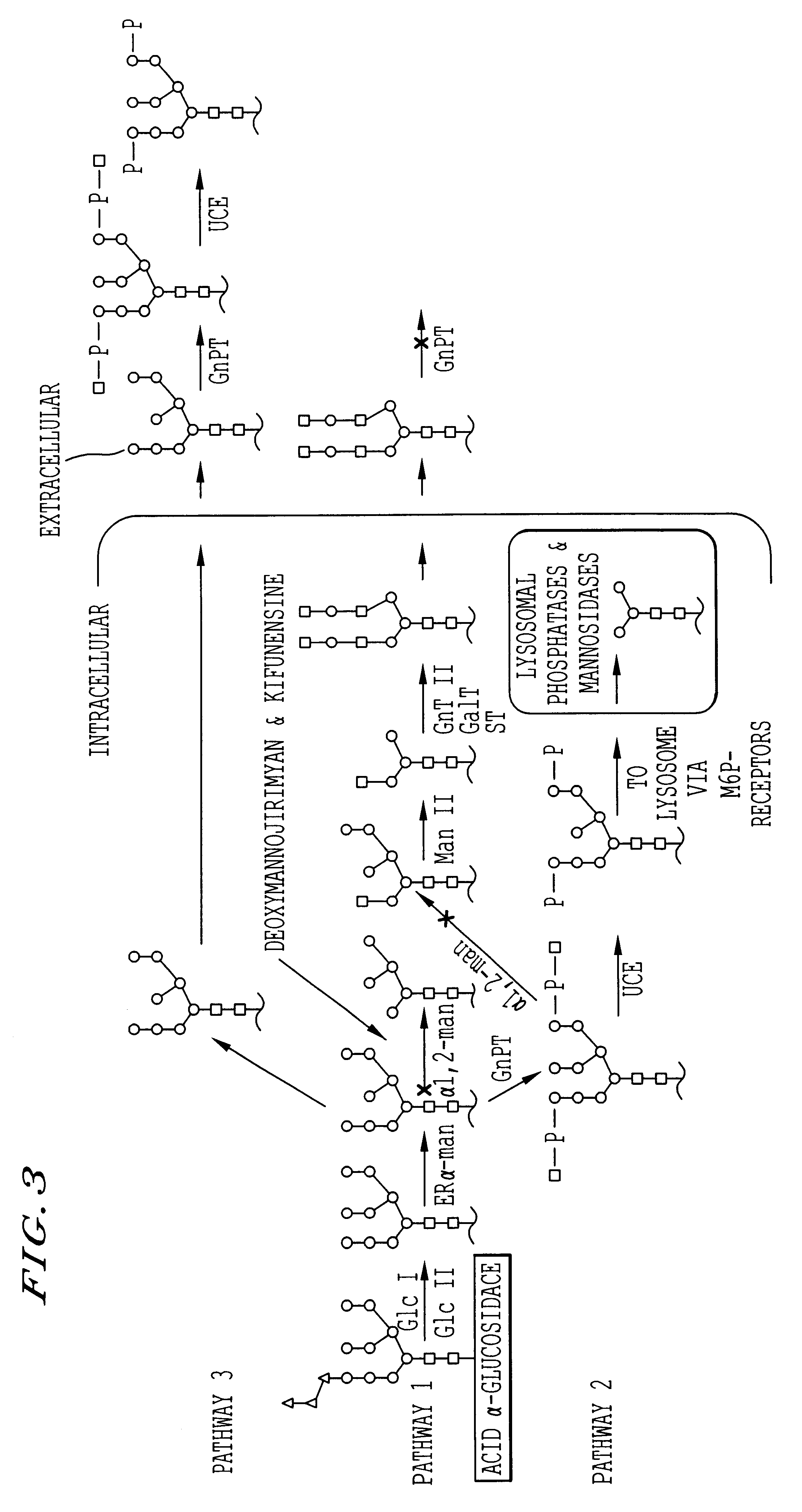
Methods for producing highly phosphorylated lysosomal hydrolases
InactiveUS6534300B1Easy to identifyHigh mannose structureFungiBacteriaLysosomal targetingPhosphorylation
The present invention provides highly phosphorylated lysosomal hydrolases, methods of modifying lysosomal hydrolases with the lysosomal targeting pathway enzymes GlcNAc-phosphotransferase and / or phosphodiester alpha-GlcNAcase.
Owner:GENZYME CORP
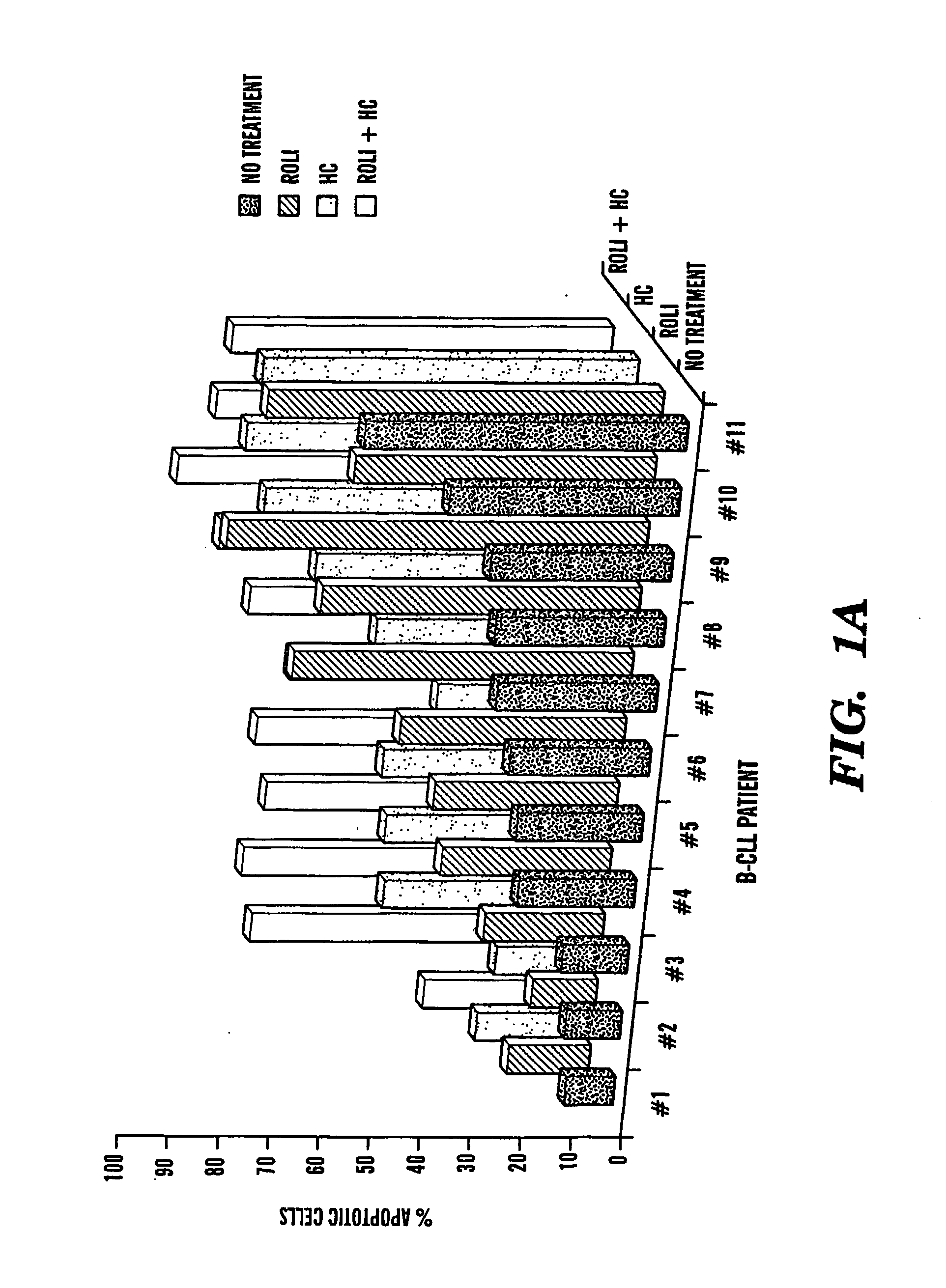
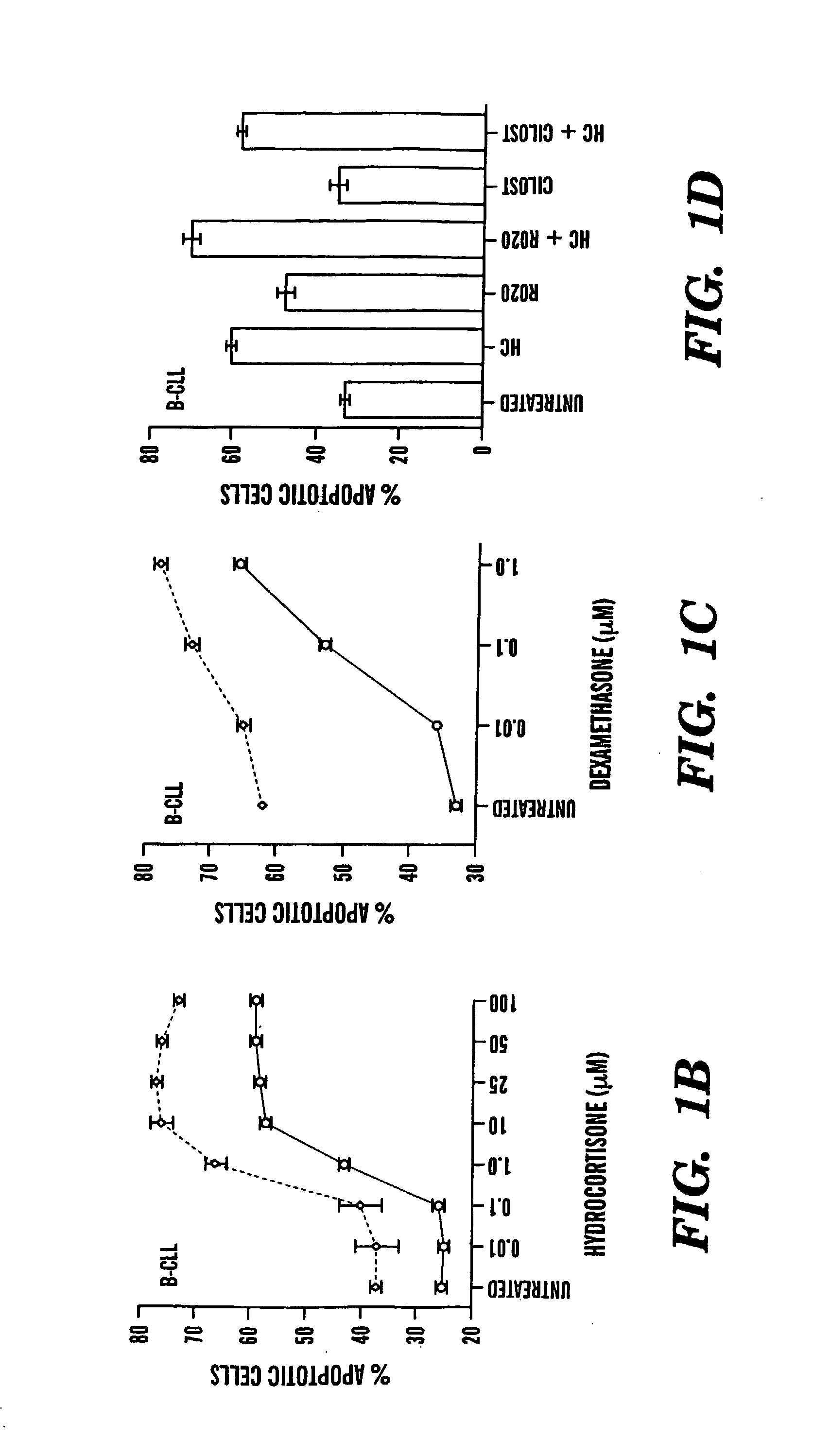
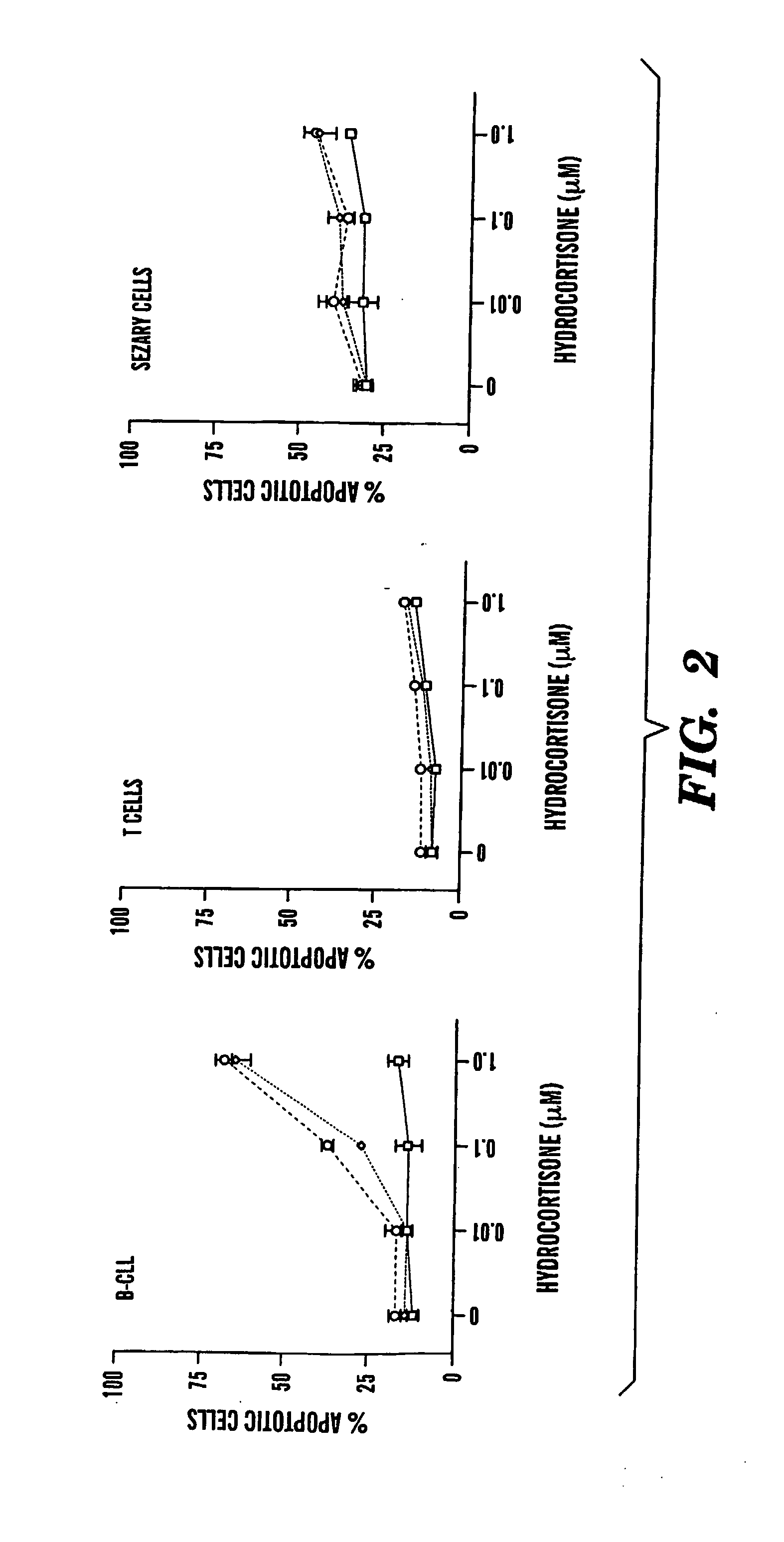
Compositions and Methods for the Treatment of Peripheral B-Cell Neoplasms
InactiveUS20080051379A1High level of apoptosisLevel of apoptosis inducedOrganic active ingredientsBiocidePDE4 InhibitorsAdenosine
The present invention is directed to the use of a PDE4 inhibitor and a glucocorticoid to treat peripheral B-cell neoplasms. In particular, the present invention provides a method of treating individuals (e.g. patients) diagnosed with peripheral B-cell leukemias by administering pharmaceutical compositions comprising Type 4 cyclic adenosine monophosphate phosphodiesterase inhibitors and a glucocorticoid. Preferably, the combination of the PDE4 inhibitor and the glucocorticoid has a synergistic effect on apoptosis such that the level of apoptosis induced is greater than the level that would be expected by simply adding a PDE4 inhibitor to a glucocorticoid.
Owner:BOSTON MEDICAL CENTER INC
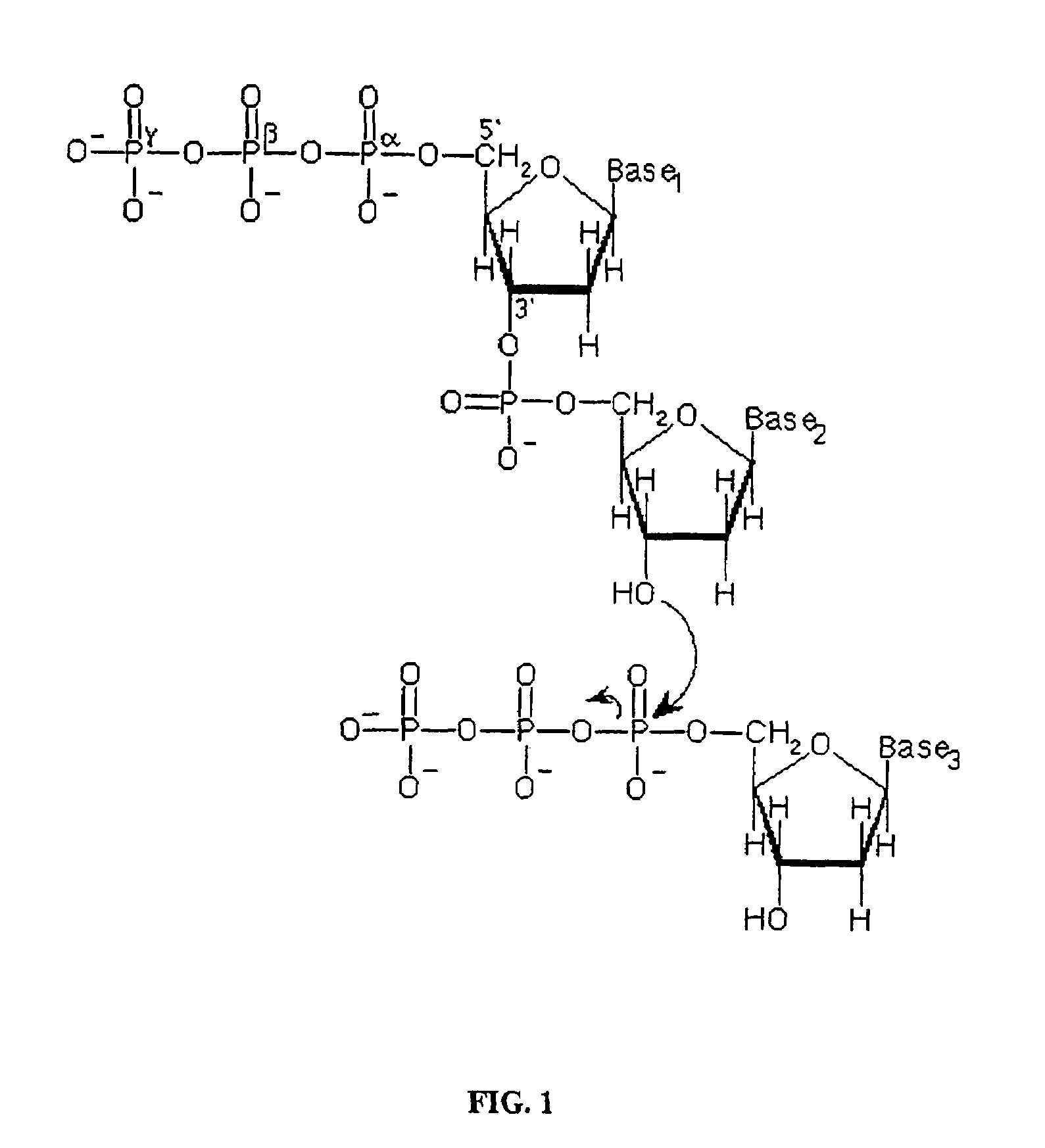
Methods of synthesizing oligonucleotides using carbonate protecting groups and alpha-effect nucleophile deprotection
The invention provides methods for synthesizing oligonucleotides using nucleoside monomers having carbonate protected hydroxyl groups that are deprotected with α-effect nucleophiles. The α-effect nucleophile irreversibly cleave the carbonate protecting groups while simultaneously oxidizing the internucleotide phosphite triester linkage to a phosphodiester linkage. The procedure may be carried out in aqueous solution at neutral to mildly basic pH. The method eliminates the need for separate deprotection and oxidation steps, and, since the use of acid to remove protecting groups is unnecessary, acid-induced depurination is avoided. Fluorescent or other readily detectable carbonate protecting groups can be used, enabling monitoring of individual reaction steps during oligonucleotide synthesis. The invention is particularly useful in the highly parallel, microscale synthesis of oligonucleotides.
Owner:AGILENT TECH INC +1
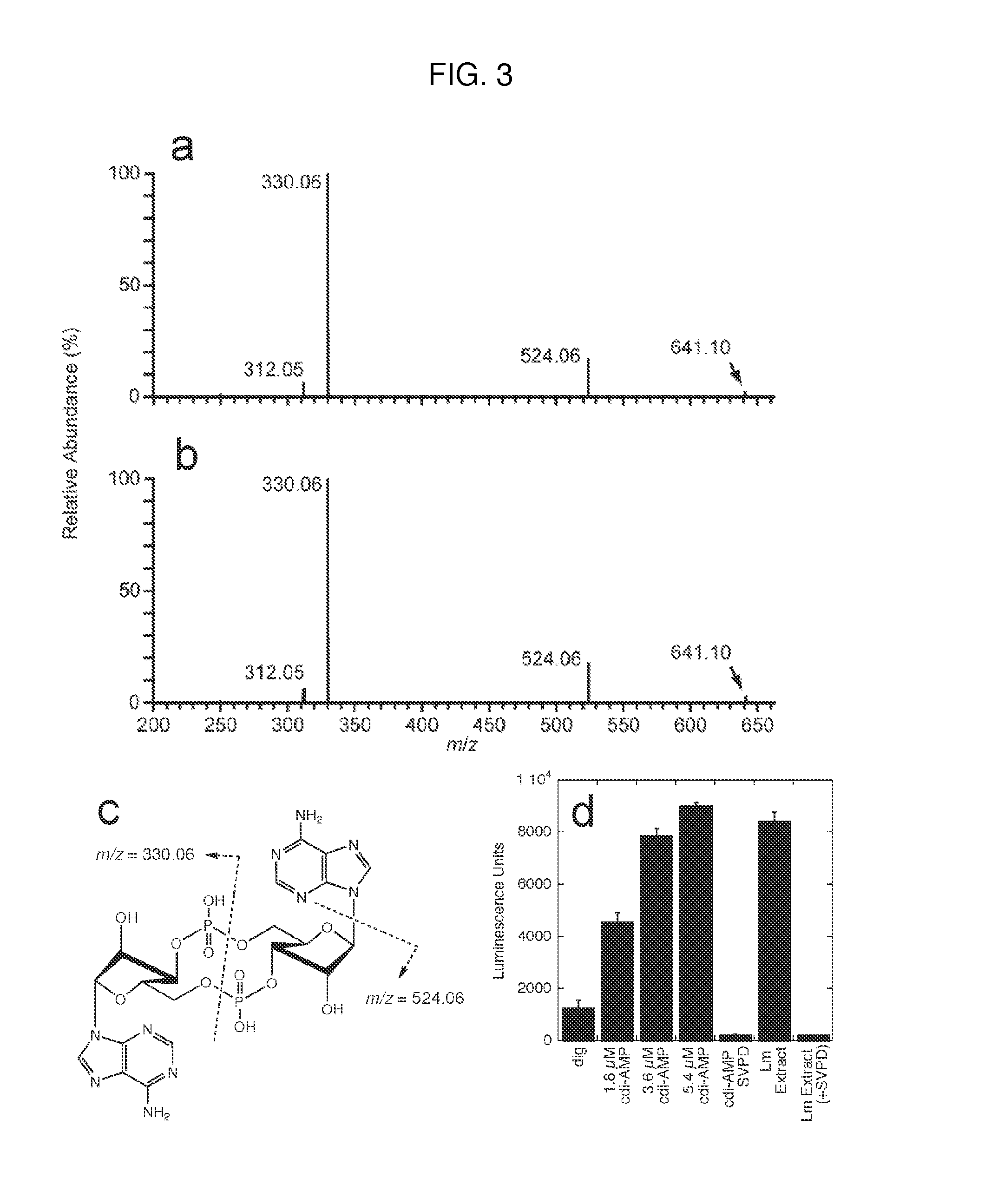
Cyclic di-amp induction of type i interferon
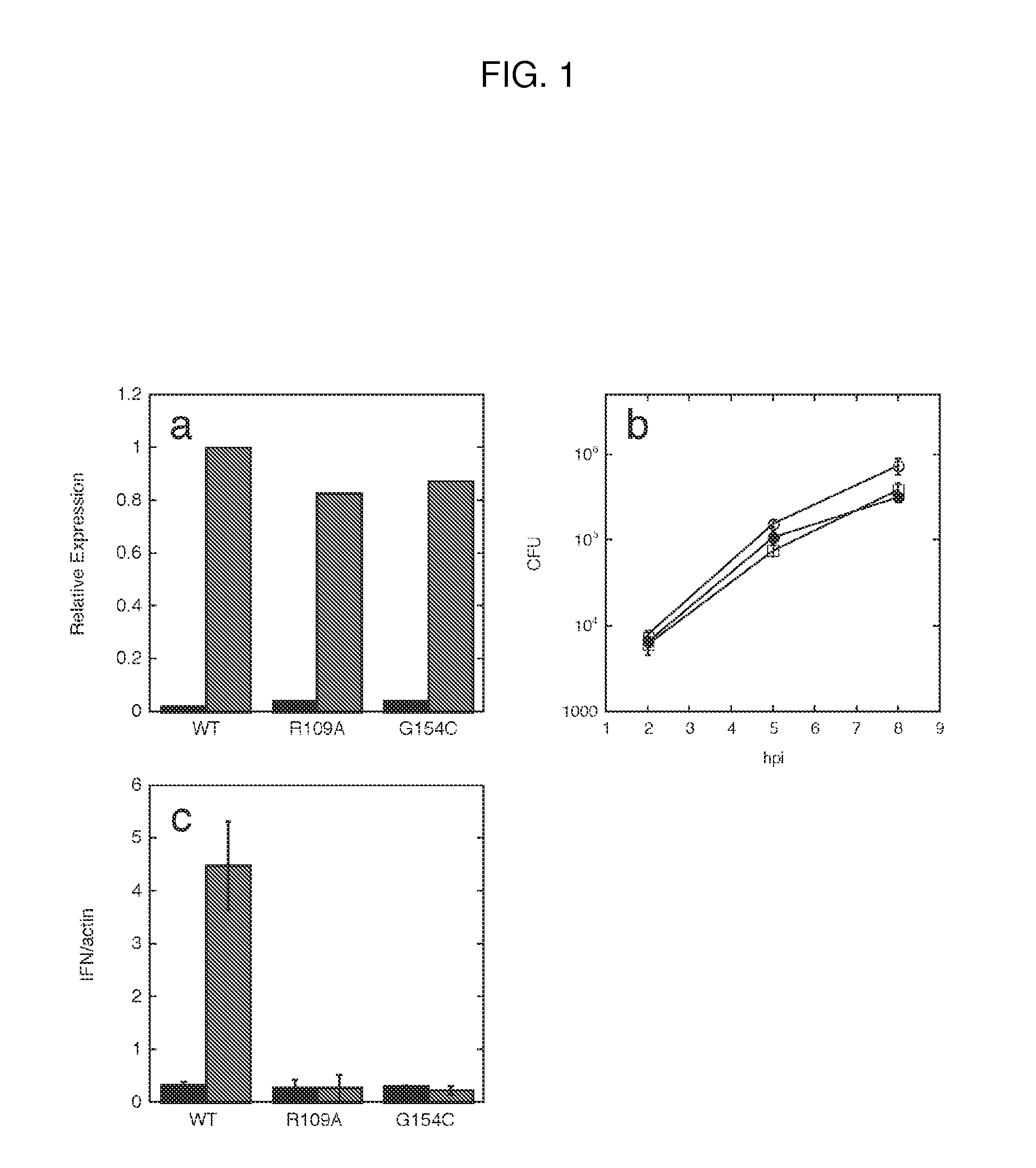
InactiveUS20120164107A1Increase secretionDecrease c-di-AMP phosphodiesterase activityBiocideBacteriaCyclaseProtein composition
Methods of modulating type-I interferon production in a cell are provided. Aspects of the methods include modulating cytosolic cyclic di-adenosine monophosphate (c-di-AMP) activity in the cell in a manner sufficient to modulate type-I interferon production in the cell. Additional aspects of the invention include c-di-AMP activity modulatory compositions, e.g., c-di-AMP, mutant Listeria bacteria, cyclase and / or phosphodiesterase nucleic acid or protein compositions, etc. The subject methods and compositions find use in a variety of applications, including therapeutic applications.
Owner:RGT UNIV OF CALIFORNIA
Method of nucleic acid sequence determination
ActiveUS20170314072A1Unable to formatMicrobiological testing/measurementTransferasesNucleotideFluorescence
Provided are sequencing-by-binding methods of detecting cognate nucleotides using a crippled DNA polymerizing enzyme that possesses the ability to bind the next correct nucleotide downstream of a primer in a template-dependent fashion, but does not possess the activity needed to promote phosphodiester bond formation. Use of the crippled DNA polymerase permits interrogation of one nucleotide at a time, without incorporation of any nucleotide. Labeled nucleotides, such as fluorescently labeled nucleotides, can be used in conjunction with the crippled DNA polymerase to establish cognate nucleotide identity in a rapid manner.
Owner:PACIFIC BIOSCIENCES
Novel uses
InactiveUS20100323997A1Enhancement of signaling pathwayAmeliorate any conditionOrganic active ingredientsBiocideSexual functioningContragestazol
The present invention relates to a new use for compounds that inhibit phosphodiesterase 1 (PDE1), e.g., that inhibit PDE1-mediated suppression of the dopamine D1 receptor and / or progesterone signaling pathways, including, e.g., methods of treatment or prophylaxis for conditions which may be ameliorated by enhancing the progesterone signaling response, particularly female sexual dysfunction.
Owner:INTRA CELLULAR THERAPIES INC
Carboline derivatives as cGMP phosphodiesterase inhibitors
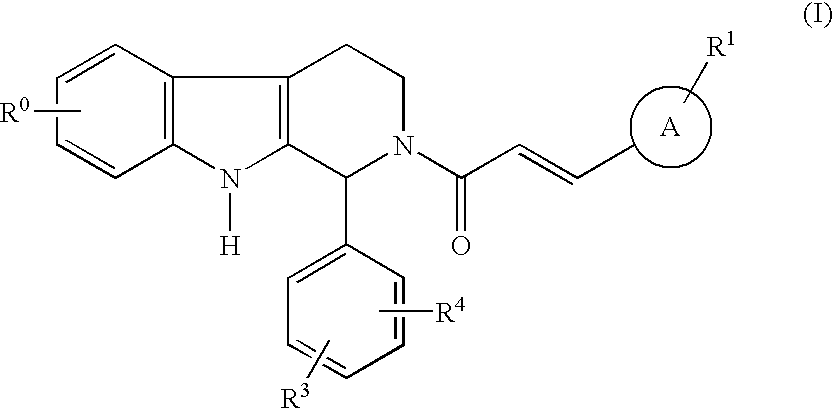
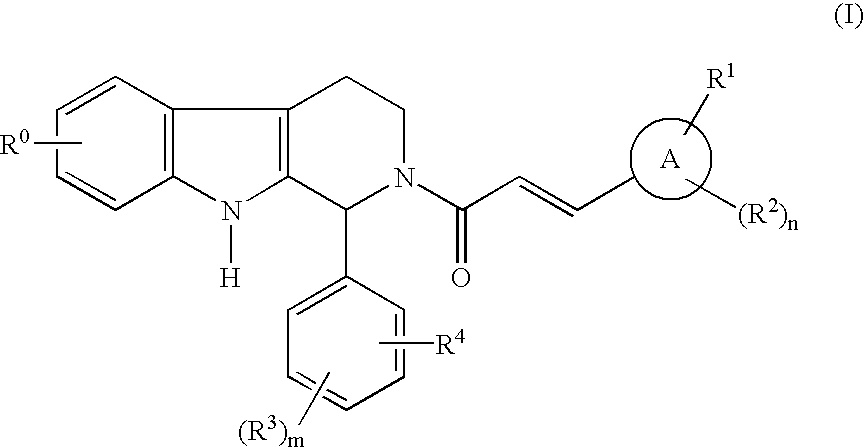
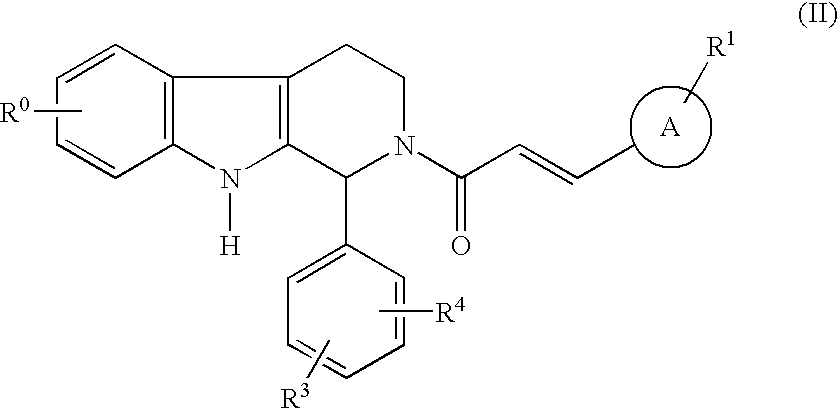
Compounds of general structural formula (I) wherein A represents a 5- or 6-membered heteroaryl group containing at least one heteroatom selected from the group consisting of oxygen, nitrogen, and sulfur, and use of the compounds, and salts and solvates thereof, as therapeutic agents, are disclosed.
Owner:ICOS CORP
Phosphodiesterase-4 inhibitor
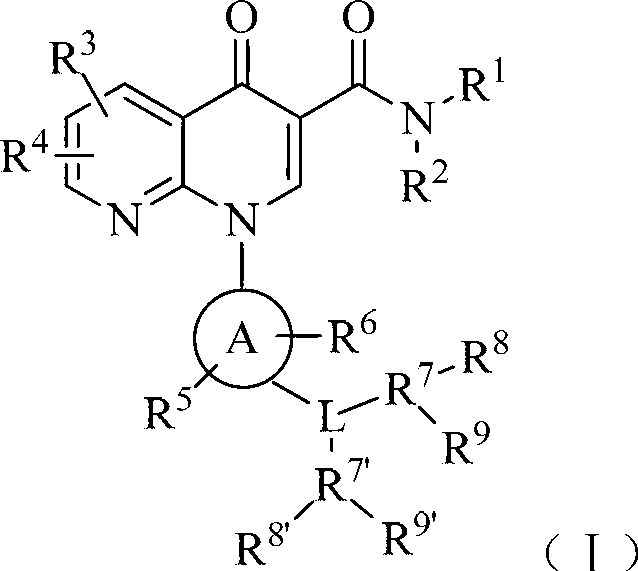
InactiveCN103183675AStrong inhibitory activityGood anti-inflammatory effectOrganic active ingredientsSenses disorderSolventInflammatory skin disease
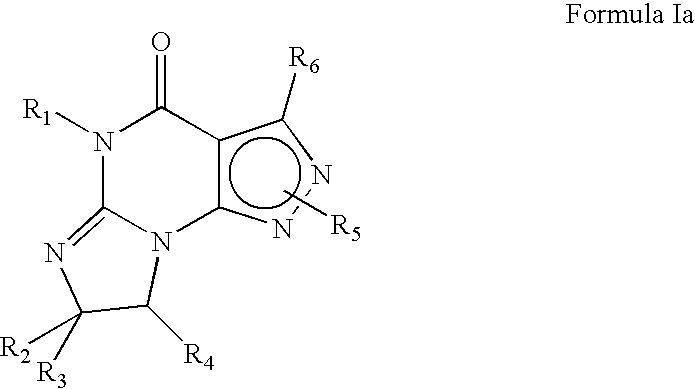
The invention belongs to the technical field of medicines and particularly relates to a phosphodiesterase-4 inhibitor as shown in a general formula (I) and a pharmaceutically acceptable salt, a stereoisomer or a solvent compound thereof. In the formula, R1, R2, R3, R4, R5, R6, R7, R8, R9, R7', R8', R9', L and a ring A are as defined in the specification. The invention further relates to a preparation method for the compound, a pharmaceutical composition with the compound, and an application of the compound and the pharmaceutical composition to preparation of drugs for treating and / or preventing inflammatory diseases, disease symptoms and disease conditions characterized by or related with undesired inflammatory immune reaction and all diseases induced by or related with TNF-alpha (tumor necrosis factor-alpha) and PDE-4 (phosphodiesterase-4) hypersecretion.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Inhibitors of cyclic amp phosphodiesterases
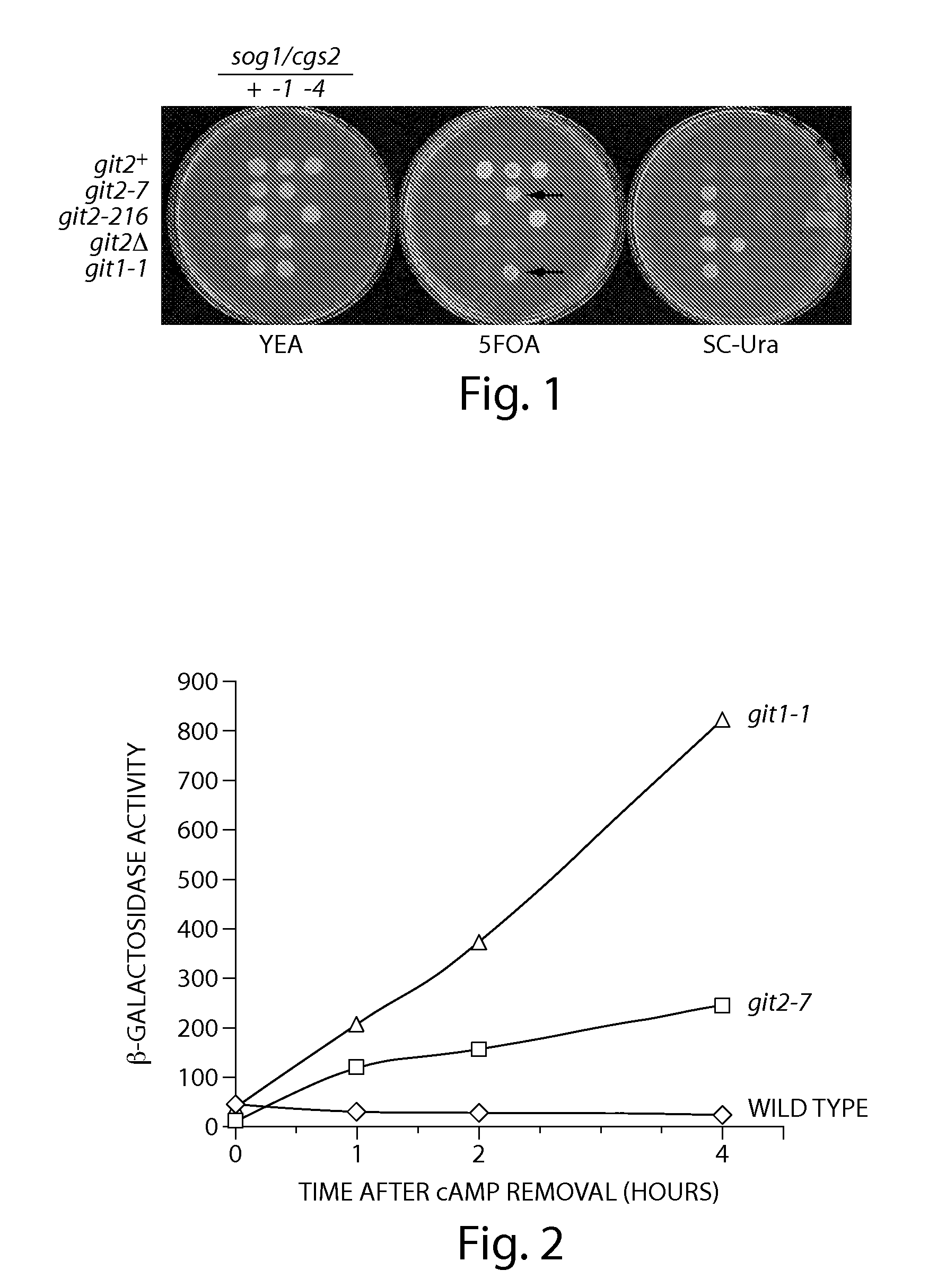
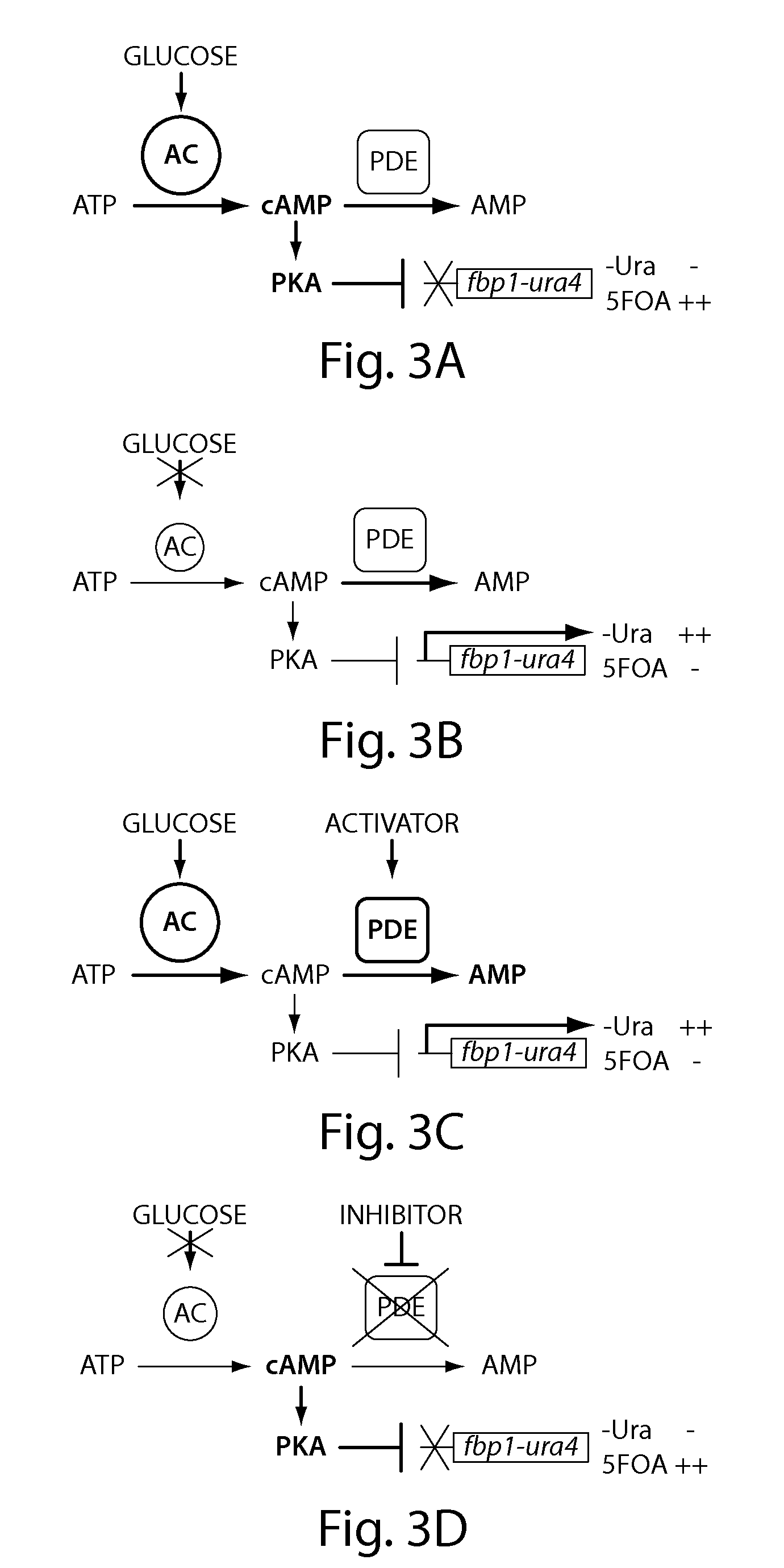
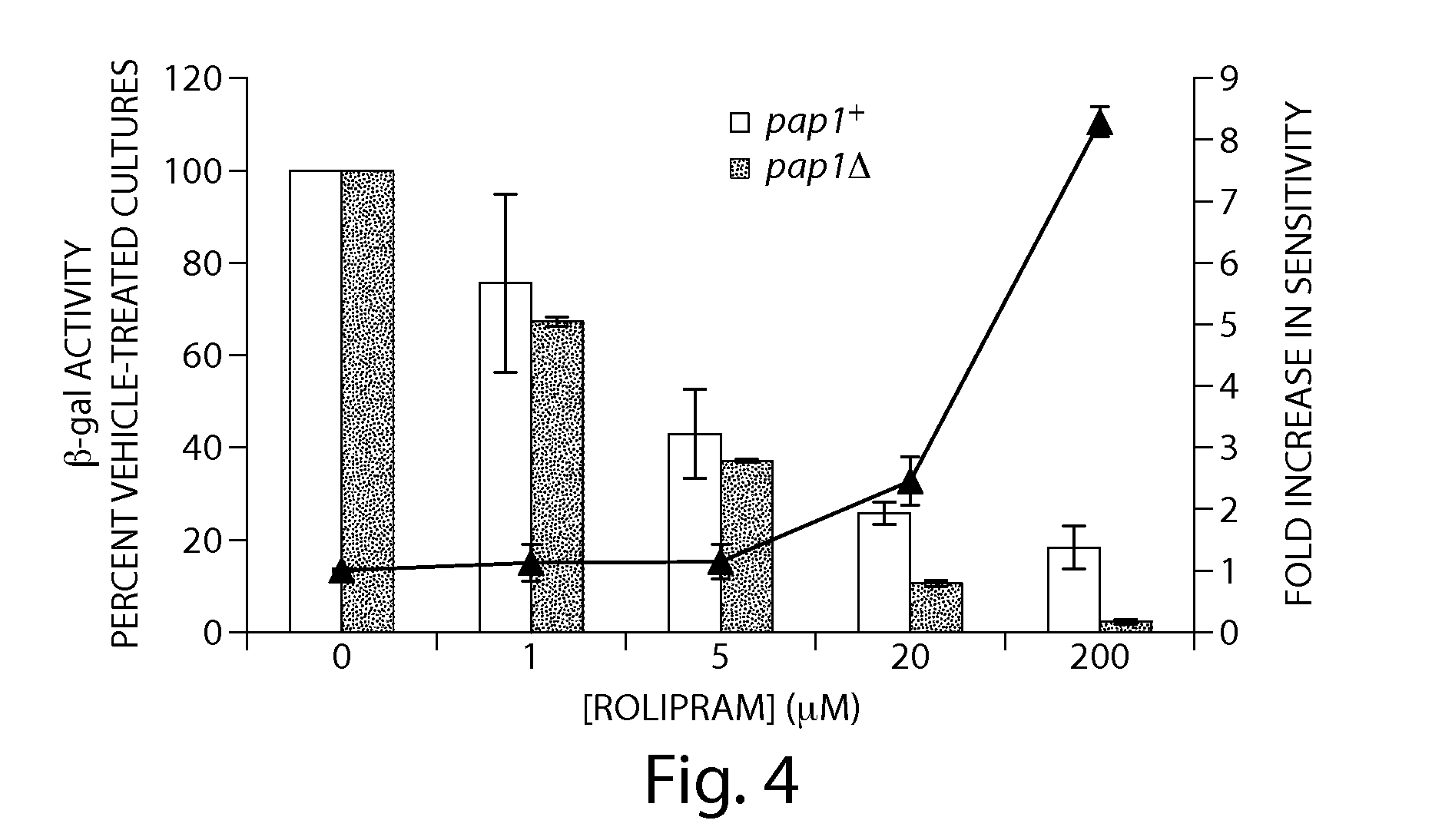
Recombinant fission yeast cells and methods of using them are described, which provide for identification of chemical and biological inhibitors or activators of a target exogenous phosphodiesterase (PDE). The invention provides, in some aspects, compounds that inhibit cAMP PDE activity and compositions that include such compounds. The invention, in part, also includes methods of using cAMP PDE-inhibiting compounds in the treatment of cAMP PDE-associated diseases and / or disorders.
Owner:BOSTON COLLEGE
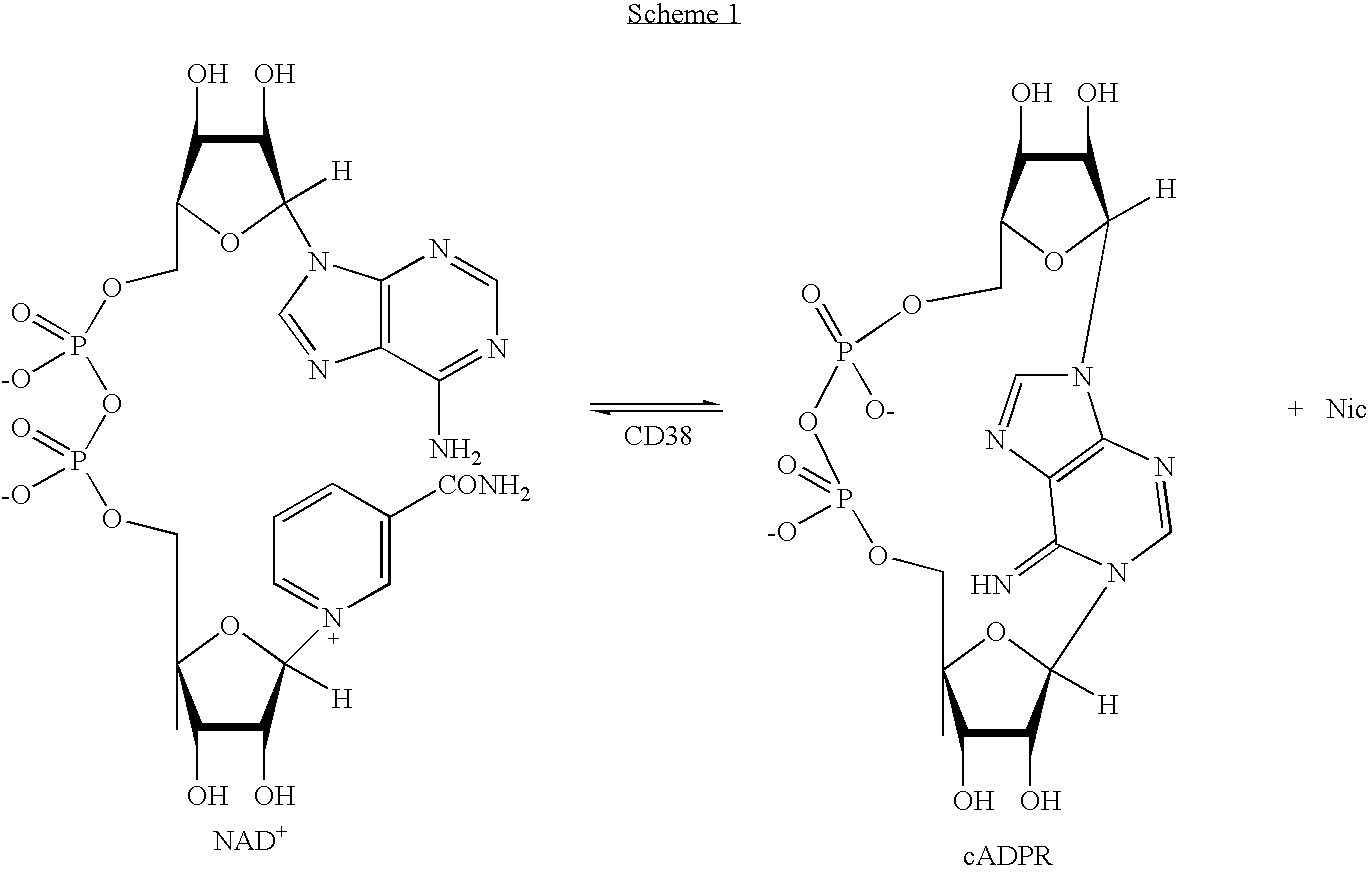
Inhibitors of ADP-ribosyl transferases, cyclases, and hydrolases
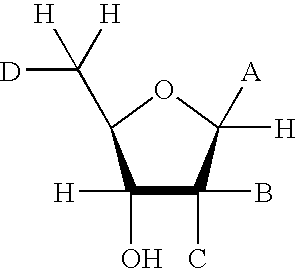
The present invention provides compounds having the formula: wherein A is a nitrogen-, oxygen-, or sulfur-linked aryl, alkyl, cyclic, or heterocyclic group, the group being further substituted with an electron contributing moiety; B is hydrogen, or a halogen, amino, or thiol group; C is hydrogen, or a halogen, amino, or thiol group; and D is a primary alcohol, a hydrogen, or an oxygen, nitrogen, carbon, or sulfur linked to phosphate, a phosphoryl group, a pyrophosphoryl group, or adenosine monophosphate through a phosphodiester or carbon-, nitrogen-, or sulfur-substituted phosphodiester bridge, or to adenosine diphosphate through a phosphodiester or carbon-, nitrogen-, or sulfur-substituted pyrophosphodiester bridge. The present invention also provides pharmaceutical compositions containing the above compounds, methods of using the above compounds as pharmaceuticals, and processes for preparing the above compounds. Also provided are methods for inhibiting an ADP-ribosyl transferase, ADP-ribosyl cyclase, ADP-ribosyl hydrolase, or NAD-dependent deacetylase enzyme, and methods for treating a disease or condition associated with an ADP-ribosyl transferase, ADP-ribosyl cyclase, ADP-ribosyl hydrolase, or NAD-dependent deacetylase enzyme in a subject in need of treatment thereof.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
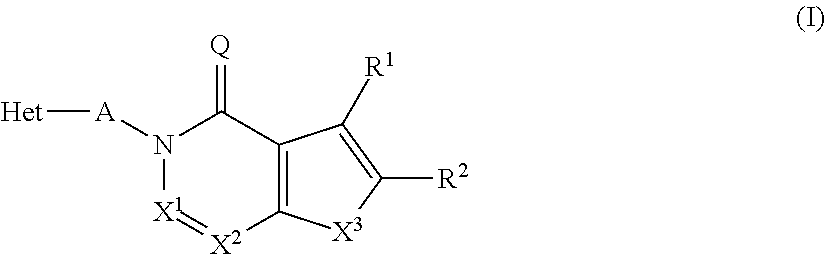
Novel inhibitor compounds of phosphodiesterase type 10a
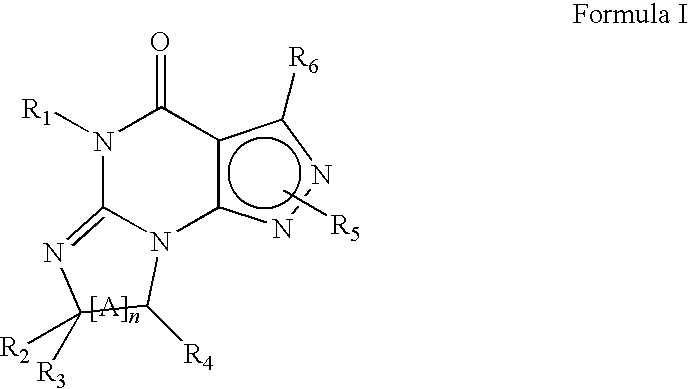
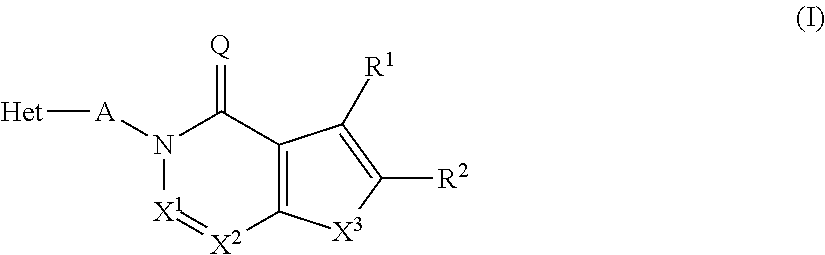
InactiveUS20130116241A1High selectivityGood metabolic stabilityBiocideNervous disorderDiseaseMedical disorder
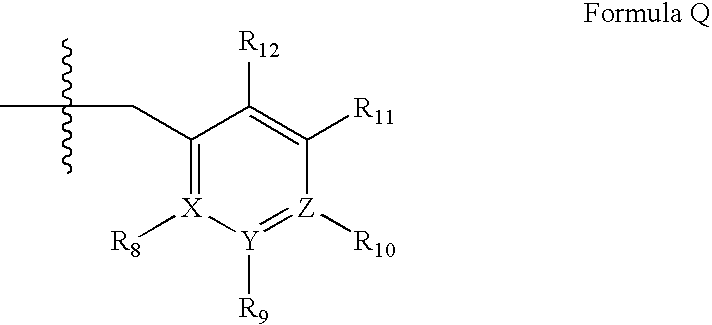
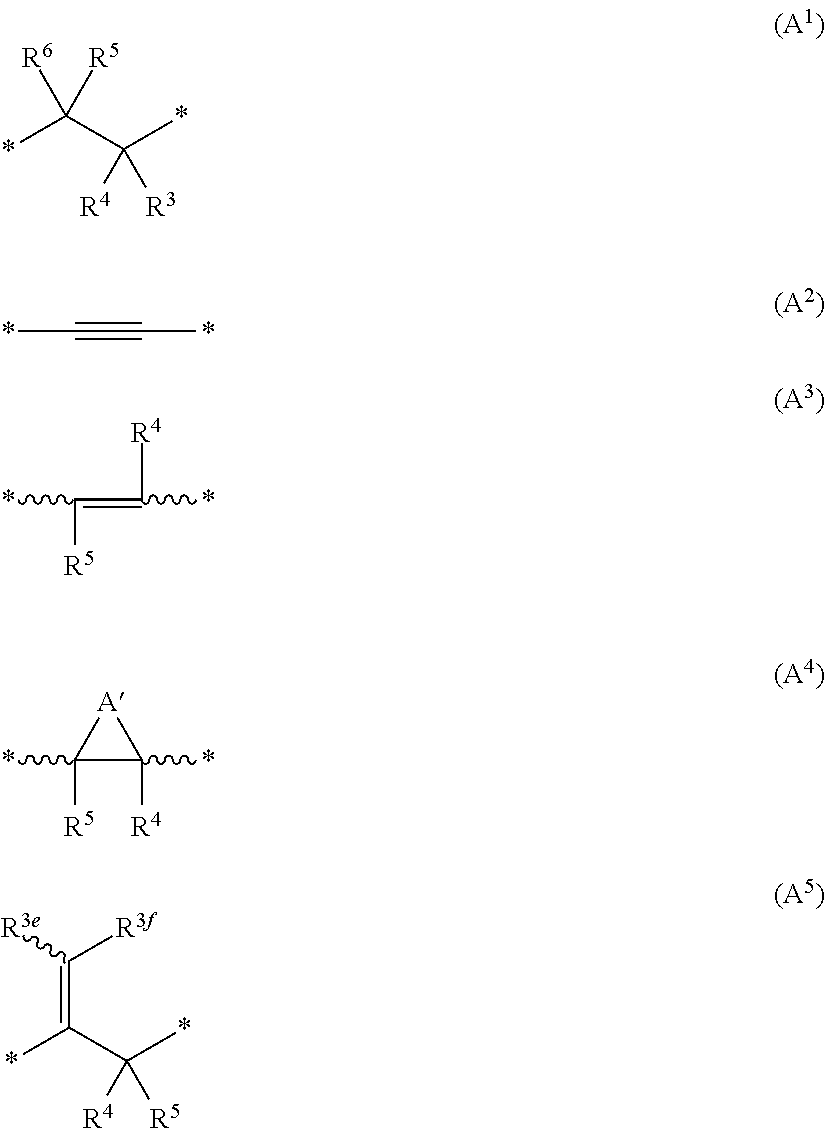
The present invention relates to novel compounds of the formula I which are inhibitors of phosphodiesterase type 10A and to their use for the manufacture of a medicament and which thus are suitable for treating or controlling of medical disorders selected from neurological disorders and psychiatric disorders, for ameliorating the symptoms associated with such disorders and for reducing the risk of such disorders.whereinQ is O or S, X1 is N or CH, X2 is N or C—R7; X3 is O, S—X4═C(R8)—, where C(R8) is bound to the carbon atom which carries R2, or —X5═C(R9)—, where X5 is bound to the carbon atom which carries R2; X4 is N or C—R9; X5 is N;Het is selected from optionally substituted phenyl, monocyclic hetaryl and fused bicyclic hetaryl;R1 is selected inter alia from hydrogen, halogen, OH, C1-C4-alkyl, trimethylsilyl, C1-C4-alkylsulfanyl, C1-C4-alkoxy-C1-C4-alkyl, C1-C4-alkoxy, C1-C4-alkoxy-C1-C4-alkoxy, the moiety Y1-Cyc1;R2 is selected inter alia from hydrogen, halogen, OH, C1-C4-alkyl, trimethylsilyl, C1-C4-alkoxy-C1-C4-alkyl, C1-C4-alkoxy, C1-C4-alkoxy-C1-C4-alkoxy, C2-C4-alkenyloxy, etc;A represents one of the following groups A1, A2, A3, A4 or A5:where * indicates the points of attachment to Het and to the nitrogen atom, respectively;and where R3 to R9, R3e, R3f, A′, Y1 and Cyc1 are defined in the claims.
Owner:ABBVIE INC +1
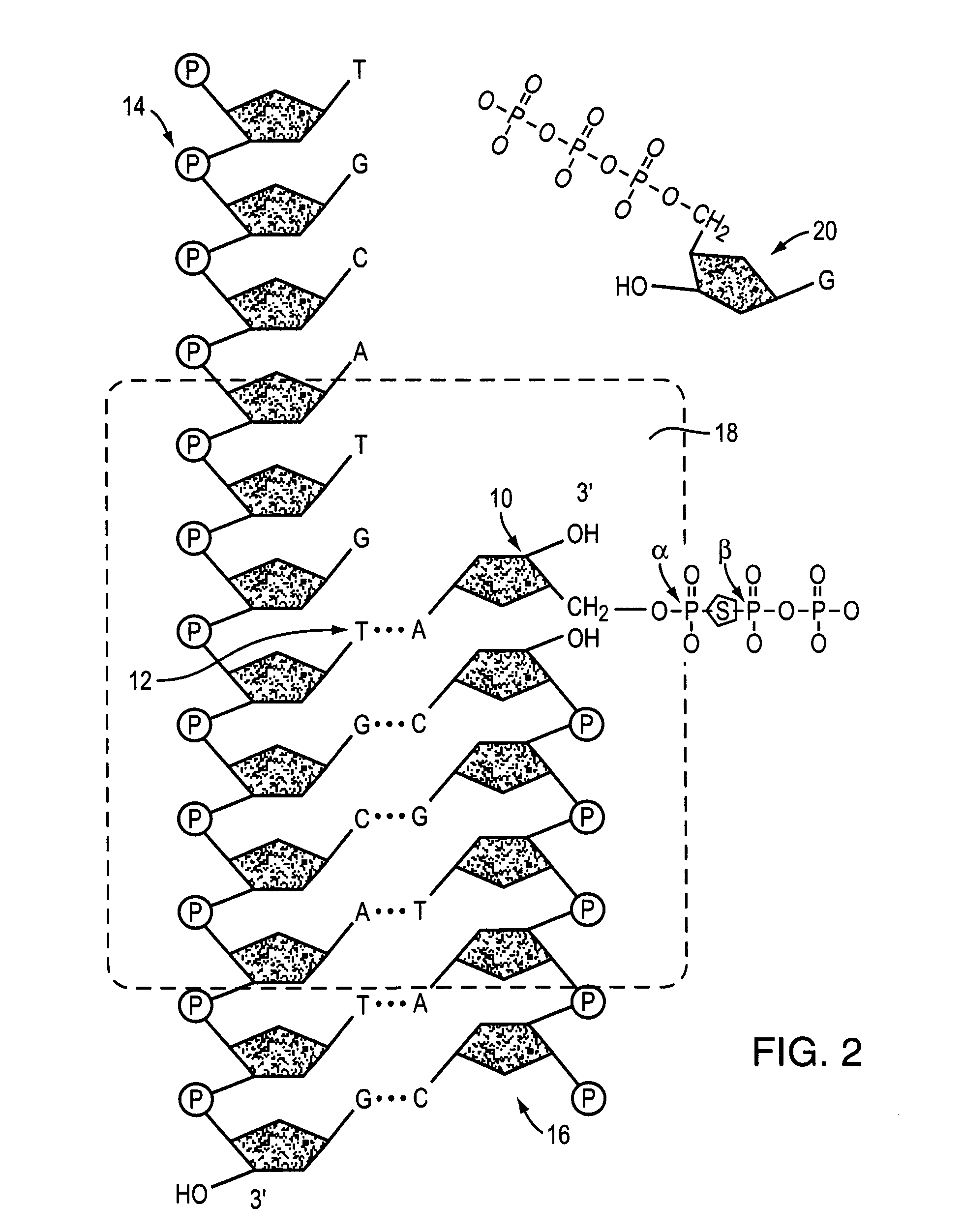
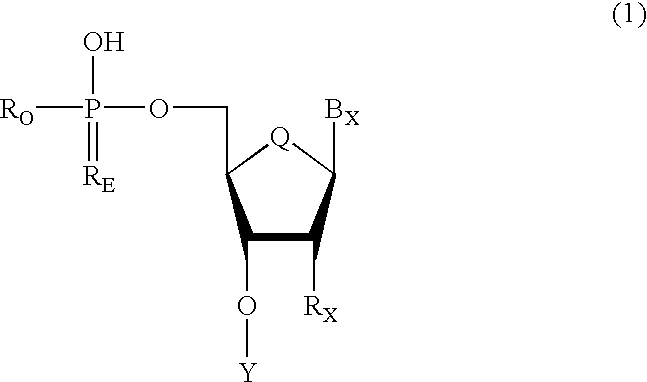
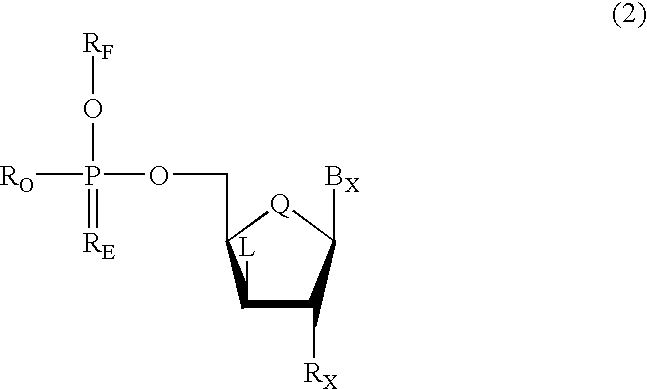
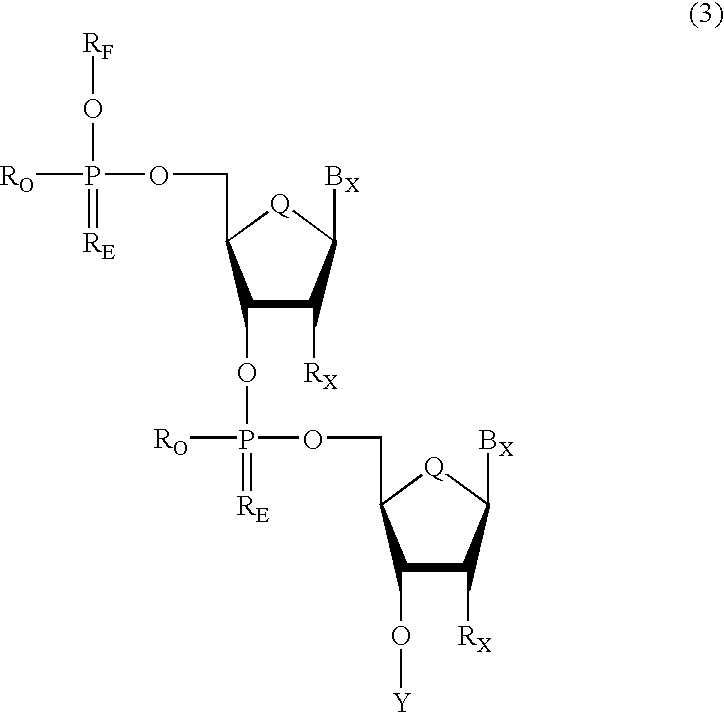
Oligonucleotides having chiral phosphorus linkages
InactiveUS20020137921A1Improved pharmacokinetic propertiesImprove propertiesSugar derivativesMicrobiological testing/measurementSugarSugar moiety
Sequence-specific oligonucleotides are provided having substantially pure chiral Sp phosphorothioate, chiral Rp phosphorothioate, chiral Sp alkylphosphonate, chiral Rp alkylphosphonate, chiral Sp phosphoamidate, chiral Rp phosphoamidate, chiral Sp phosphotriester, and chiral Rp phosphotriester linkages. The novel oligonucleotides are prepared via a stereospecific SN2 nucleophilic attack of a phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate anion on the 3' position of a xylonucleotide. The reaction proceeds via inversion at the 3' position of the xylo reactant species, resulting in the incorporation of phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate linked ribofuranosyl sugar moieties into the oligonucleotide.
Owner:IONIS PHARMA INC
7-aza-quinazoline pde10 inhibitors
The present invention is directed to 7-aza-quinazoline compounds of general structural formula Iwhich are useful as therapeutic agents for the treatment of central nervous system disorders associated with phosphodiesterase 10 (PDE10).
Owner:COX CHRISTOPHER D +3
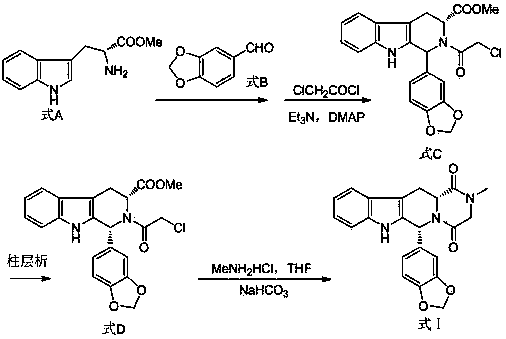
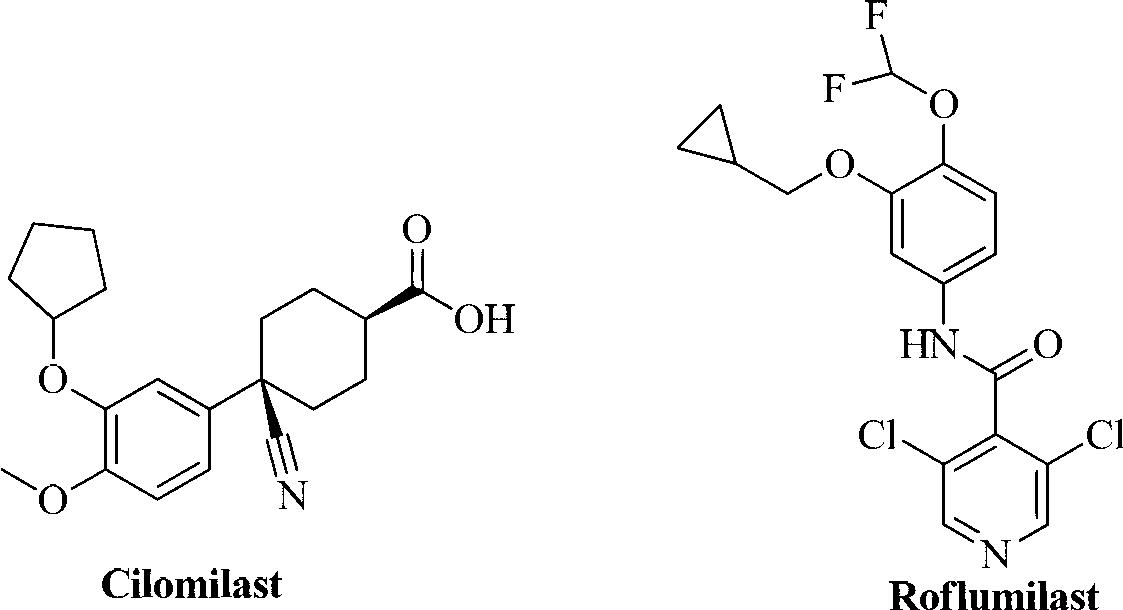
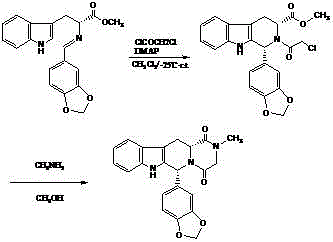
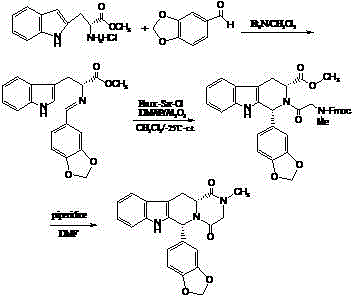
Preparing method of phosphodiesterase 5 inhibitor tadalafil
ActiveCN103980275AGood removal effectHigh chiral purityOrganic chemistryPhosphodiesterase 5 inhibitorTadalafil
The invention relates to a preparing method of a phosphodiesterase 5 inhibitor tadalafil. D-methyl tryptophanate hydrochloride is adopted as an initial raw material, and is subjected to cyclization with heliotropin, N-acylation, aminolysis-cyclization, and other reactions to obtain a tadalafil crude product. The tadalafil crude product is recrystallized to obtain a tadalafil finished product. The method has characteristics of mild reaction conditions, short reaction time, high yield, good product stability and convenience for industrial production.
Owner:湖北省医药工业研究院有限公司
Composition comprising a pde4 inhibitor and a pde5 inhibitor
InactiveUS20060094723A1Preventing and reducing onsetReduce the severity of the diseaseBiocideAntipyreticPhosphodiesterase 5 inhibitorPDE4 Inhibitors
The invention relates to the combined administration of a PDE4 inhibitor and a PDE5 inhibitor for the treatment of a disease in which phosphodiesterase 4 (PDE4) and / or phosphodiesterase 5 (PDE5) is detrimental.
Owner:NYCOMED GMBH
Process for preparing shiitake mushroom extract
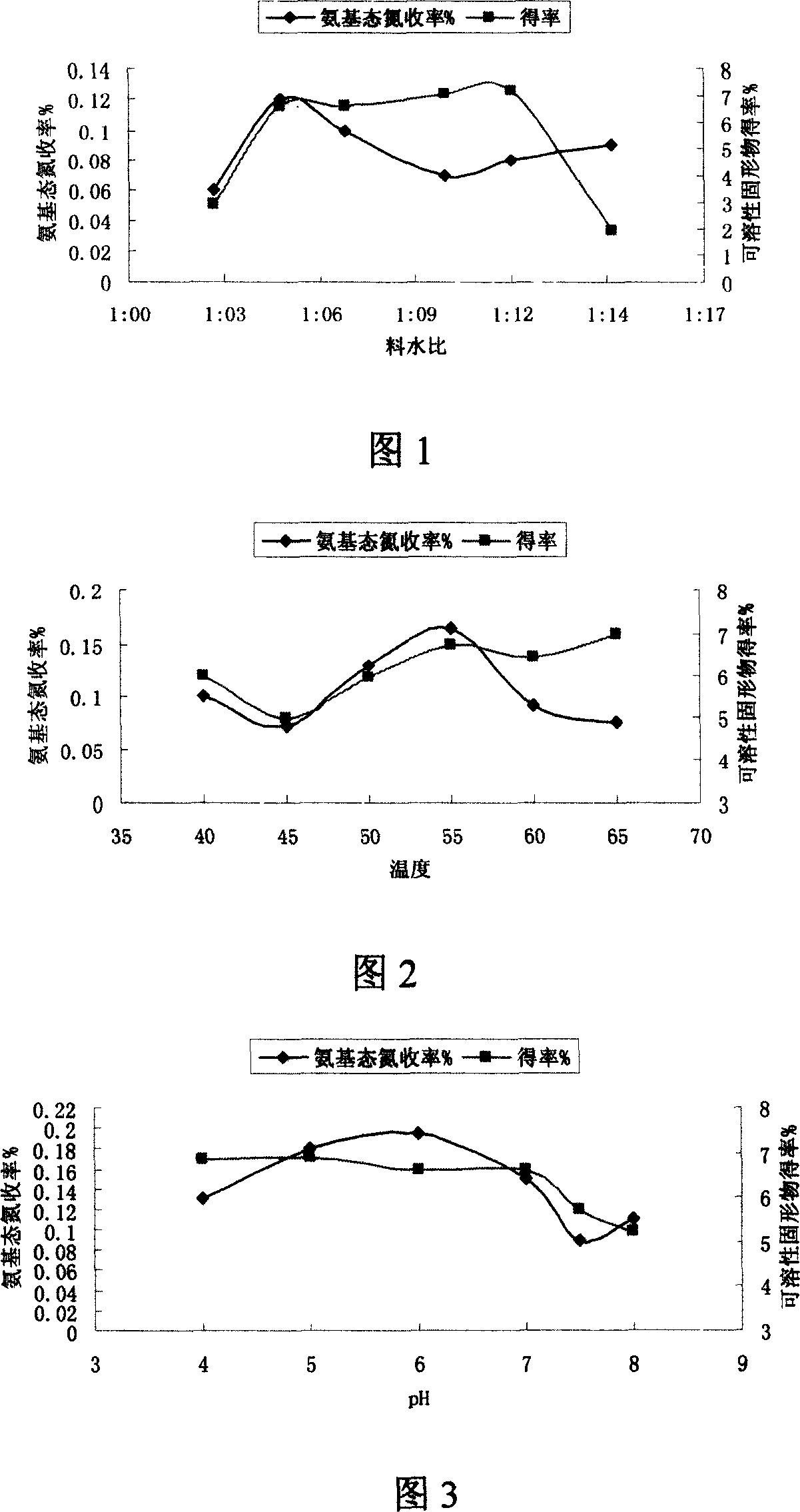
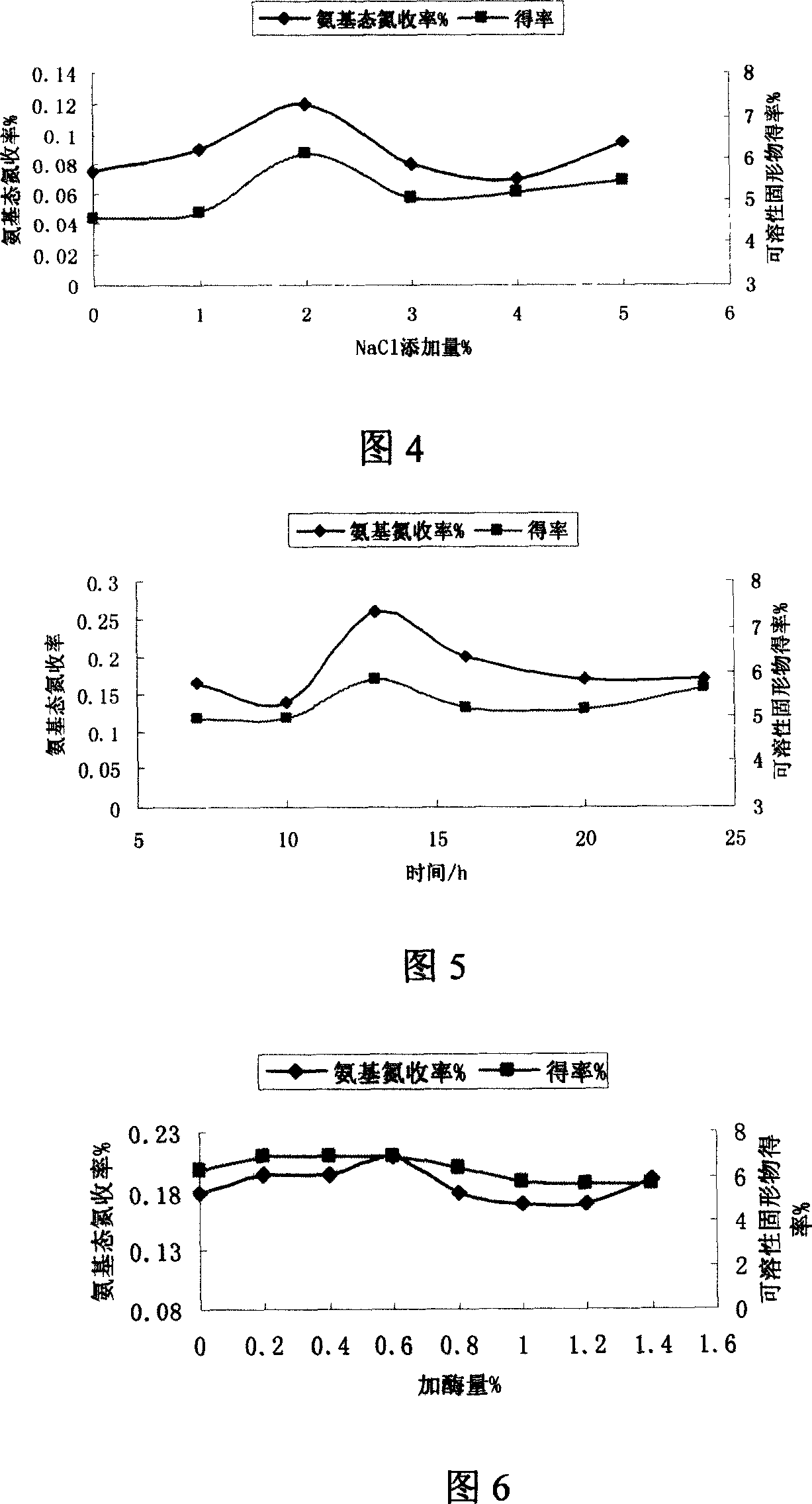
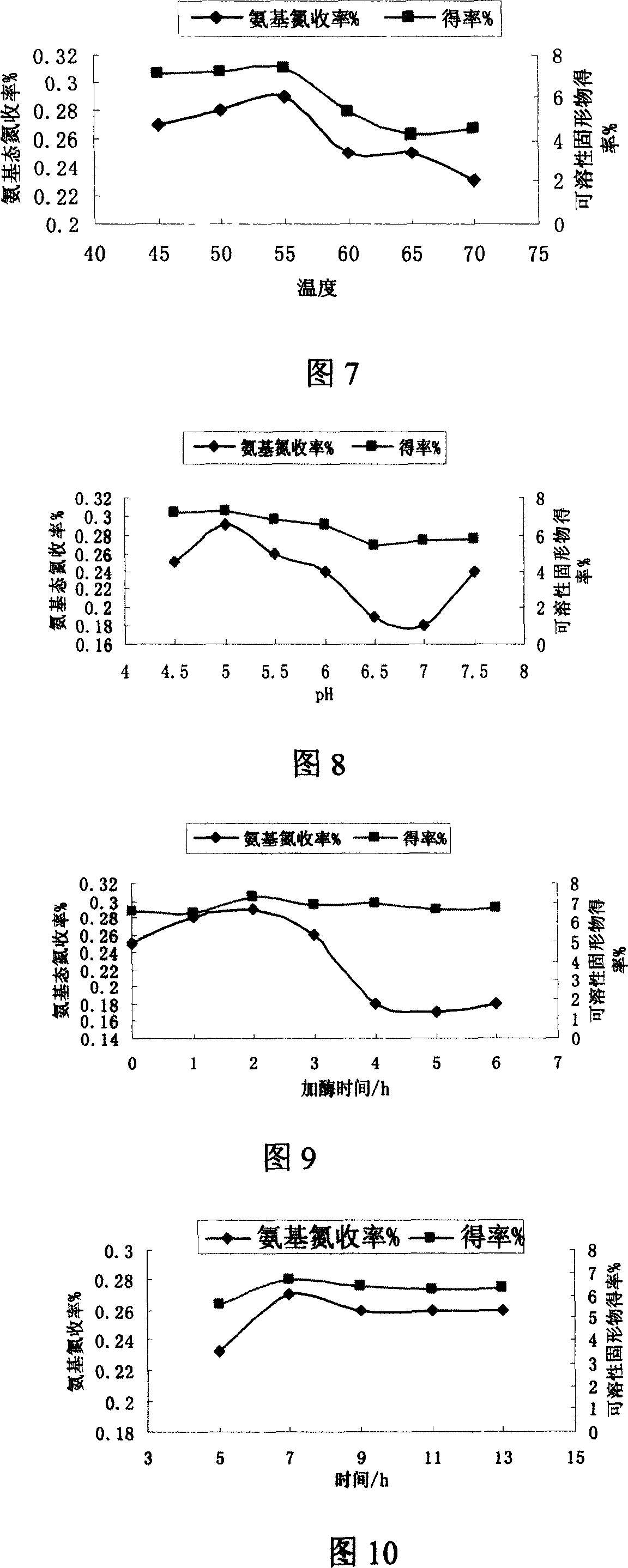
The invention relates to a method for producing mushroom, which comprises that breaking mushroom and adding water, adding common salt to dissolve for 2-20h, adding some prolease to process enzymolysis, disinfecting enzyme and separating the filter solution; adding 5'-phosphodiesterase into filter solution to process the enzymolysis, disinfecting the enzyme and separating to obtain the filter solution; concentrating the filter solution into paste or atomizing and drying into powder. The invention processes two times of enzymolysis, while the product is abundant in amino acid, polypeptide, polysaccharide, or the like. And the invention has high solubility and stability in neutral or weak-alkali water solution, and some solubility in weak-acid water solution.
Owner:郝林
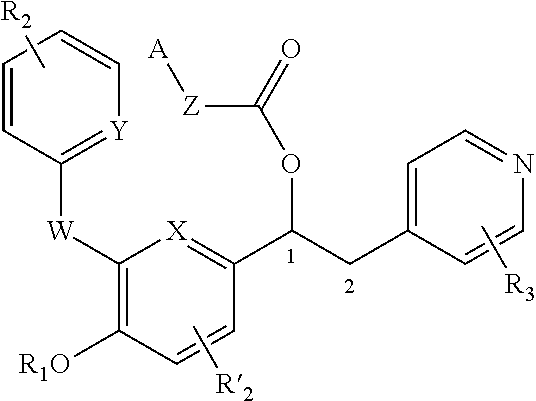
Derivatives of 1-phenyl-2-pyridinyl alkyl alcohols as phosphodiesterase inhibitors
The invention relates to inhibitors of the phosphodiesterase 4 (PDE4) enzyme. More particularly, the invention relates to compounds that are derivatives of 1-phenyl-2-pyridinyl alkyl alcohols, methods of preparing such compounds, compositions containing them and therapeutic use thereof.
Owner:CHIESI FARM SPA
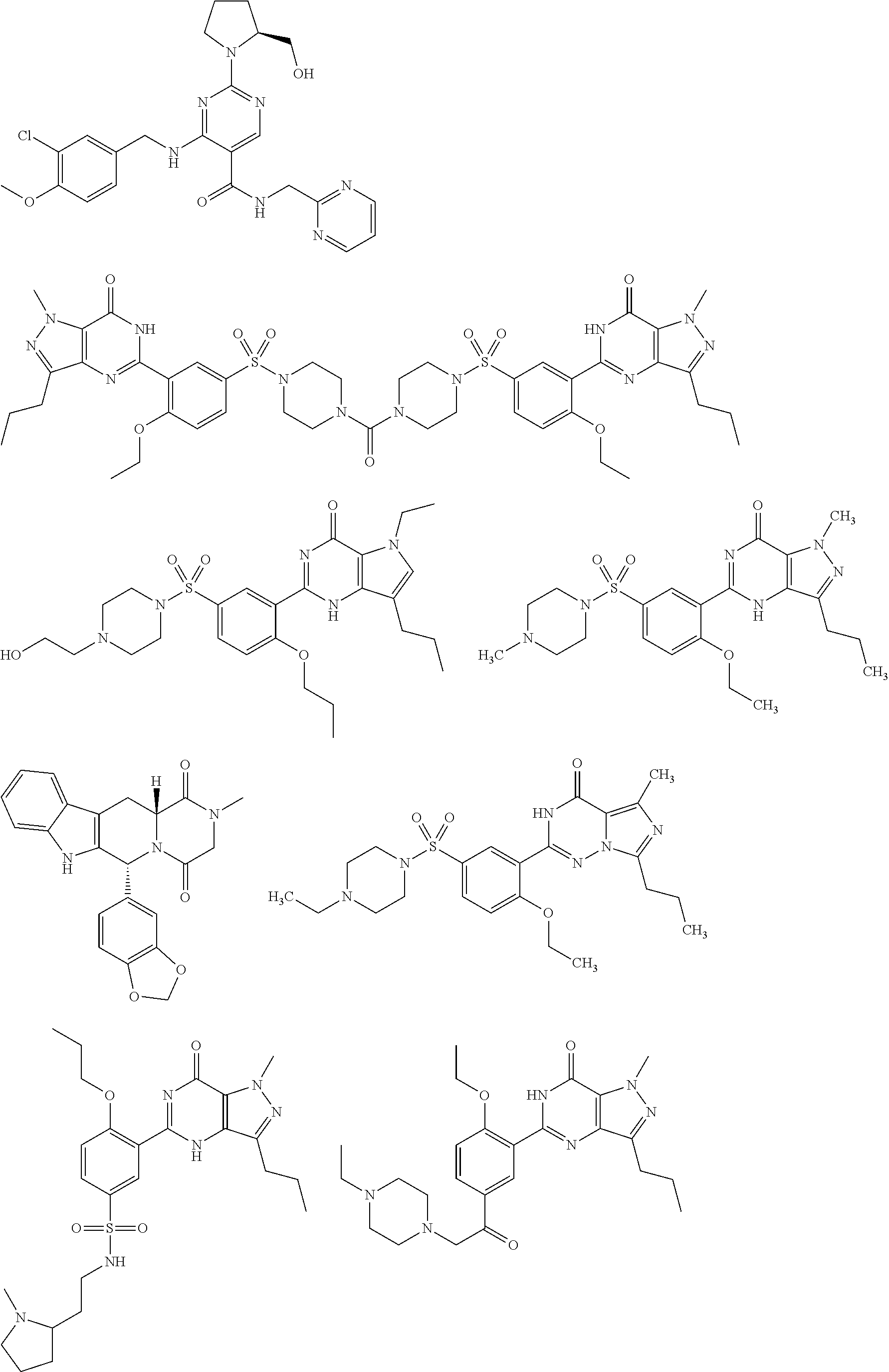
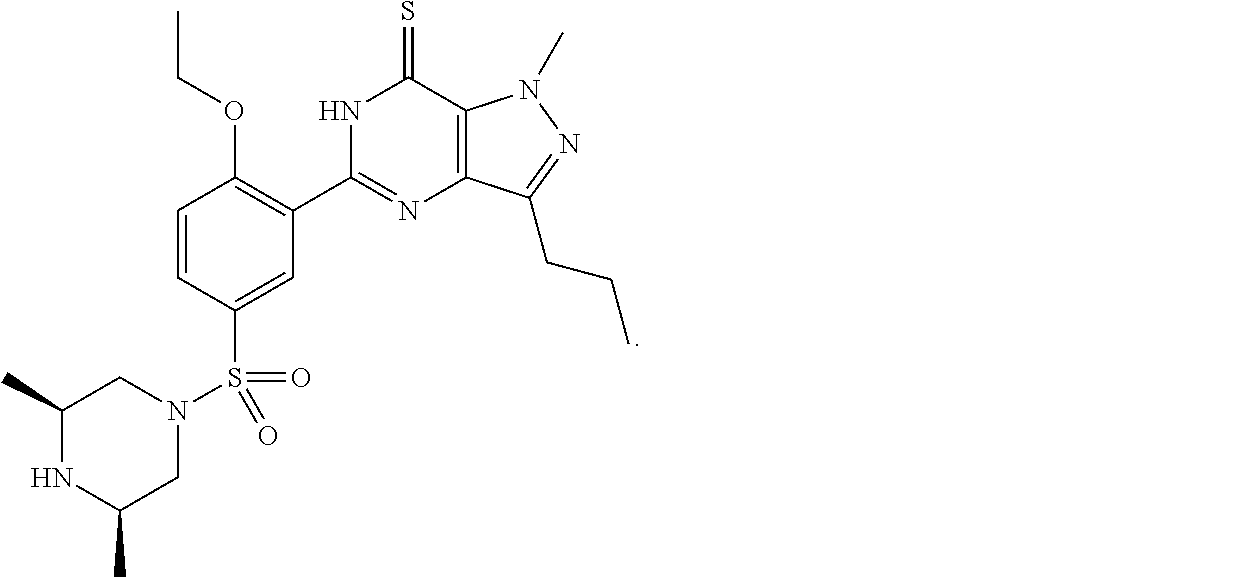
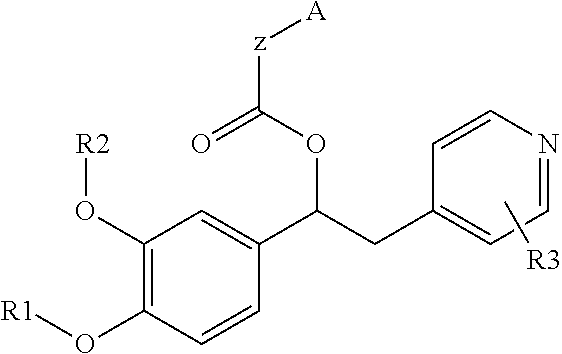
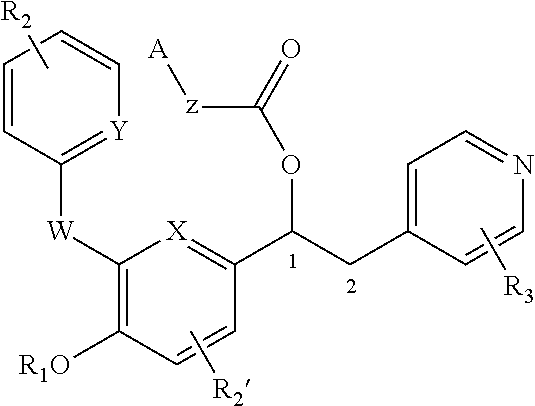
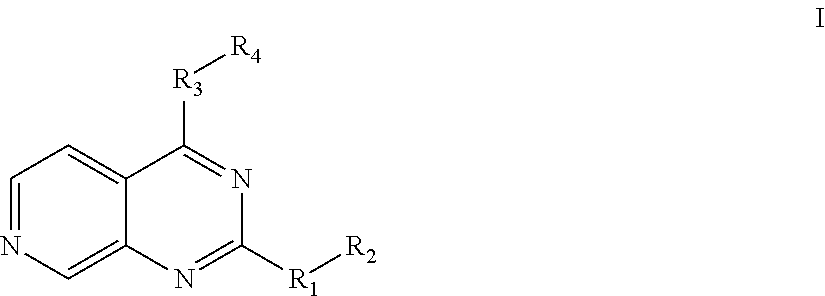
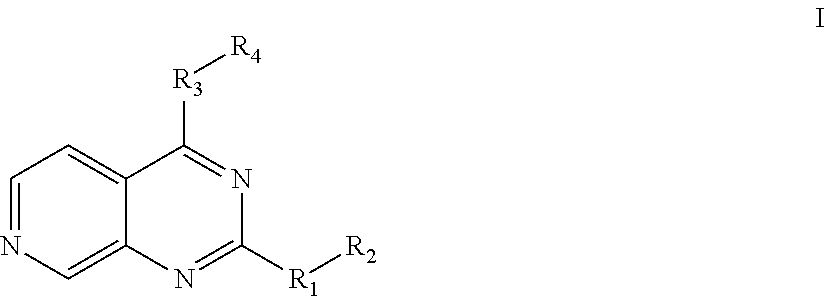
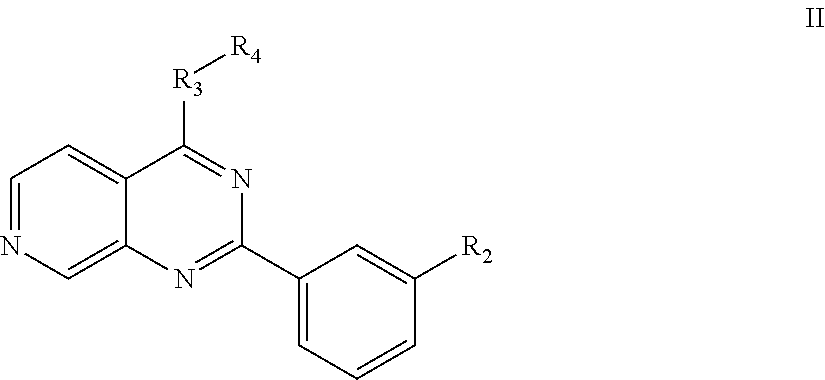
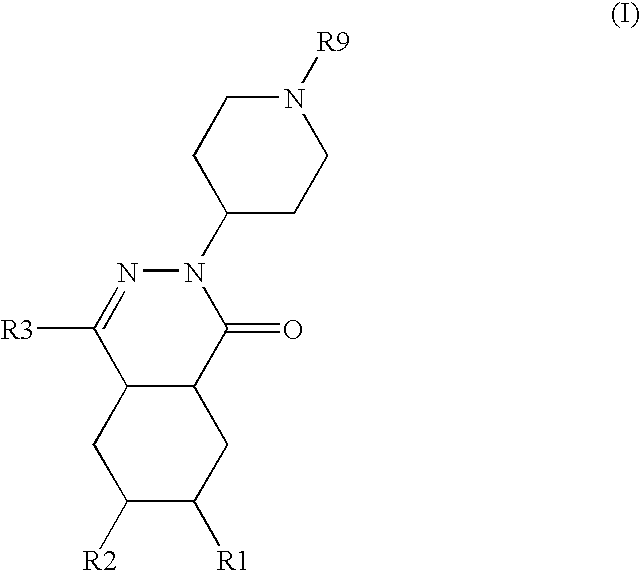
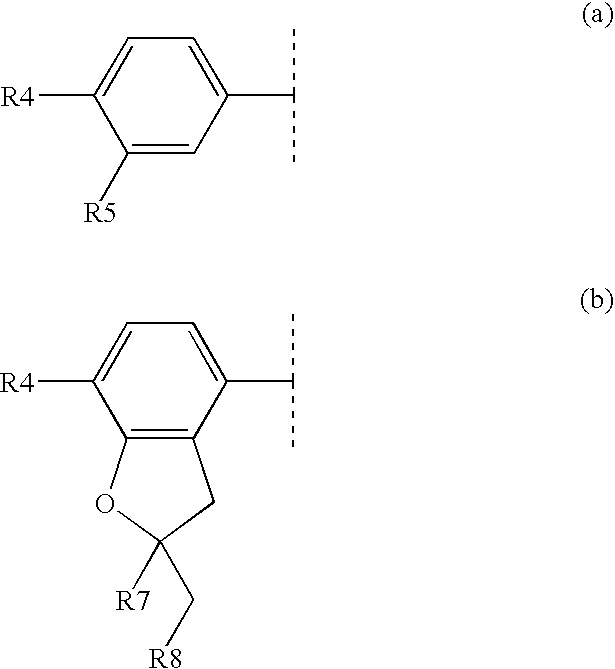
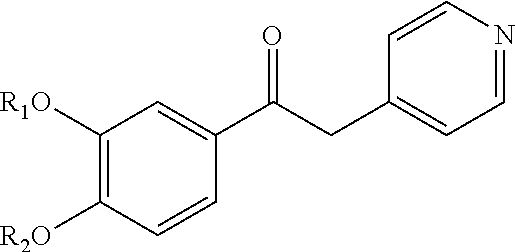
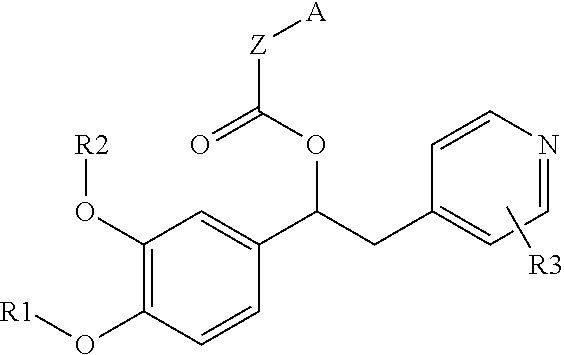
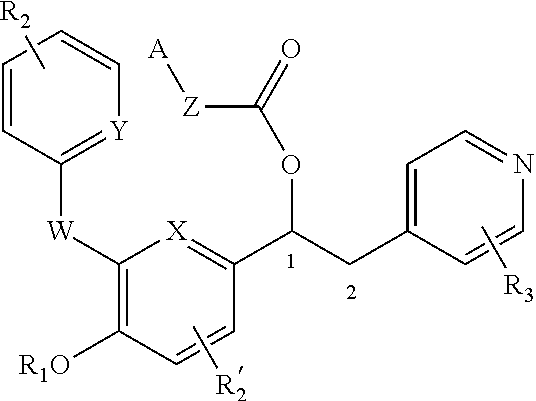
Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA
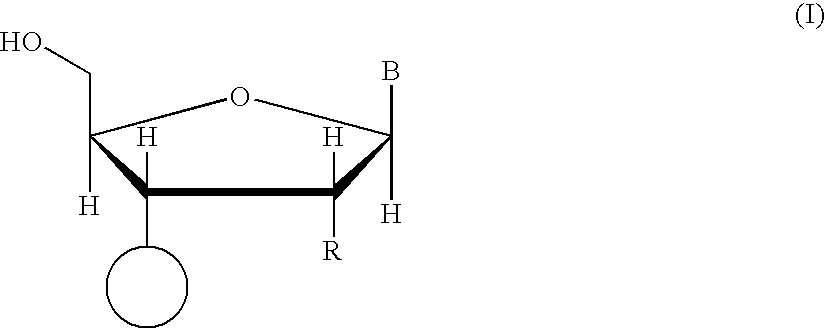
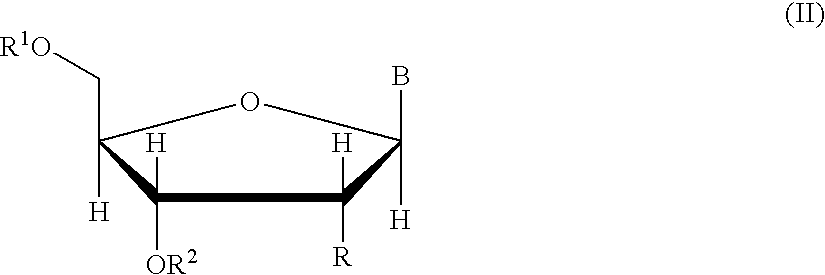
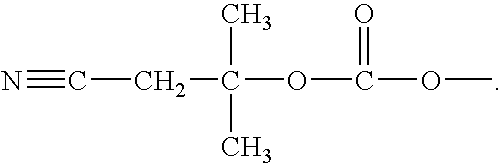
The invention relates to compounds of general formula (I); 1a, 1 b, 1 c and 1 d. The invention also relates to a method for the production thereof, pharmaceutical preparations containing said compounds and / or physiologically compatible salts thereof which can be produced therefrom and / or solvates thereof, and to the pharmaceutical use of said compounds, salts or solvates thereof as inhibitors of phosphodiesterase 4. The compounds comprise active ingredients for the treatment of diseases which can have a positive influence by inhibiting the activity of phosphodiesterase 4 and / or TNFα-release, for example, in lymphocytes, eosinophile and basophile granulocytes, macrophages and mastocytes.
Owner:THE MEDICINES CO (LEIPZIG) GMBH
Ester derivatives as phosphodiesterase inhibitors
InactiveUS7968724B2Short durationLess side effectsBiocideOrganic chemistryPhosphodiesterase inhibitorTreatment use
The invention provides inhibitors of the phosphodiesterase 4 (PDE4) enzyme. More particularly, the invention relates to compounds that are new ester derivatives, methods of preparing such compounds, compositions containing them, and therapeutic uses thereof.
Owner:CHIESI FARM SPA
Phosphodiesterase inhibitor treatment
Methods and compositions are disclosed for the treatment of taste and smell disorders. The compositions comprise phosphodiesterase inhibitors and are formulated for intranasal administration.
Owner:CYRANO THERAPEUTICS INC
Derivatives of 1-phenyl-2-pyridinyl alkyl alcohols as phosphodiesterase inhibitors
InactiveUS20130005716A1High affinityAppropriate developability profileBiocideOrganic chemistryAlcoholPhosphodiesterase inhibitor
Derivatives of 1-phenyl-2-pyridinyl alkyl alcohols are useful as inhibitors of the phosphodiesterase 4 (PDE4) enzyme and the treatment of certain conditions such as COPD.
Owner:CHIESI FARM SPA
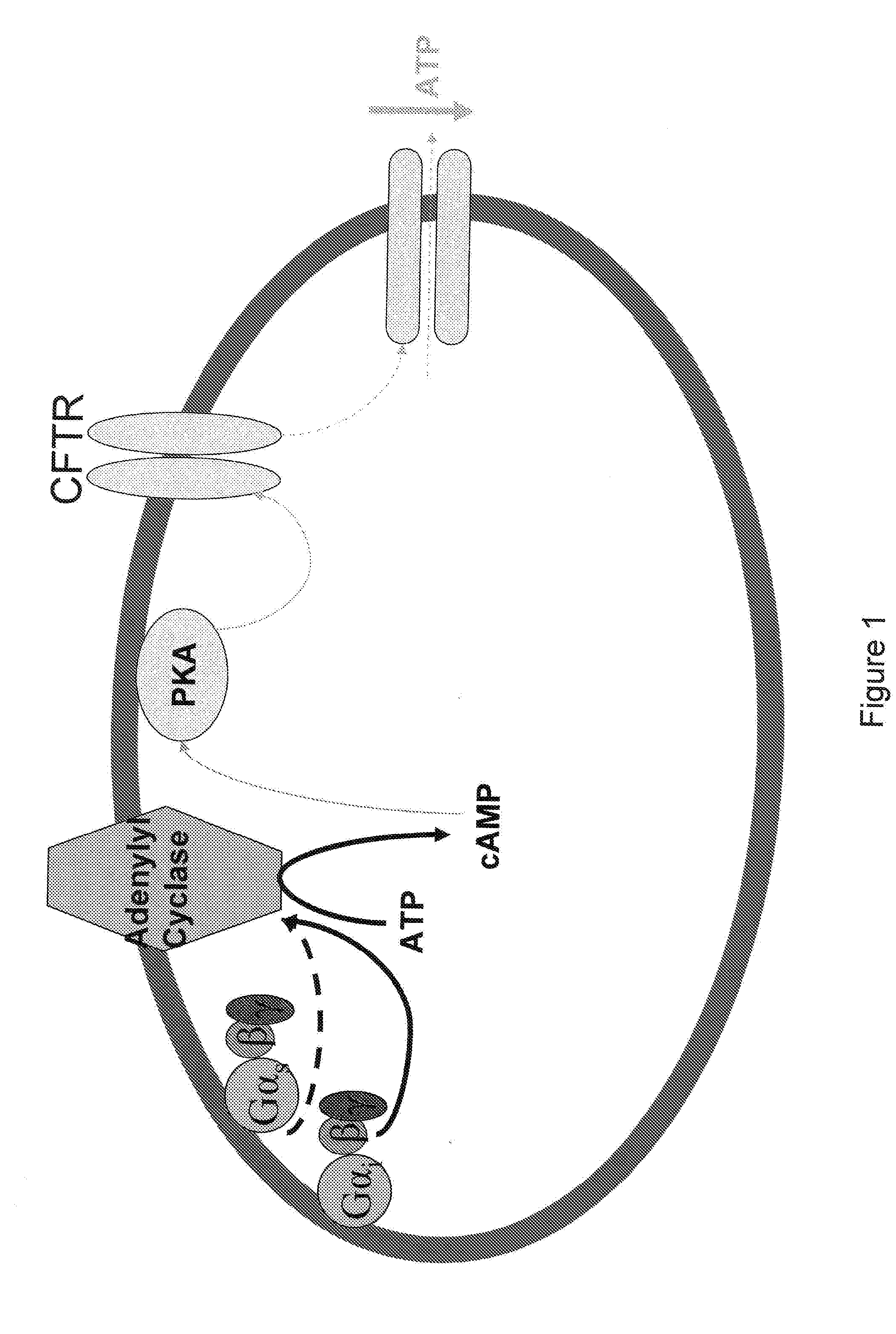
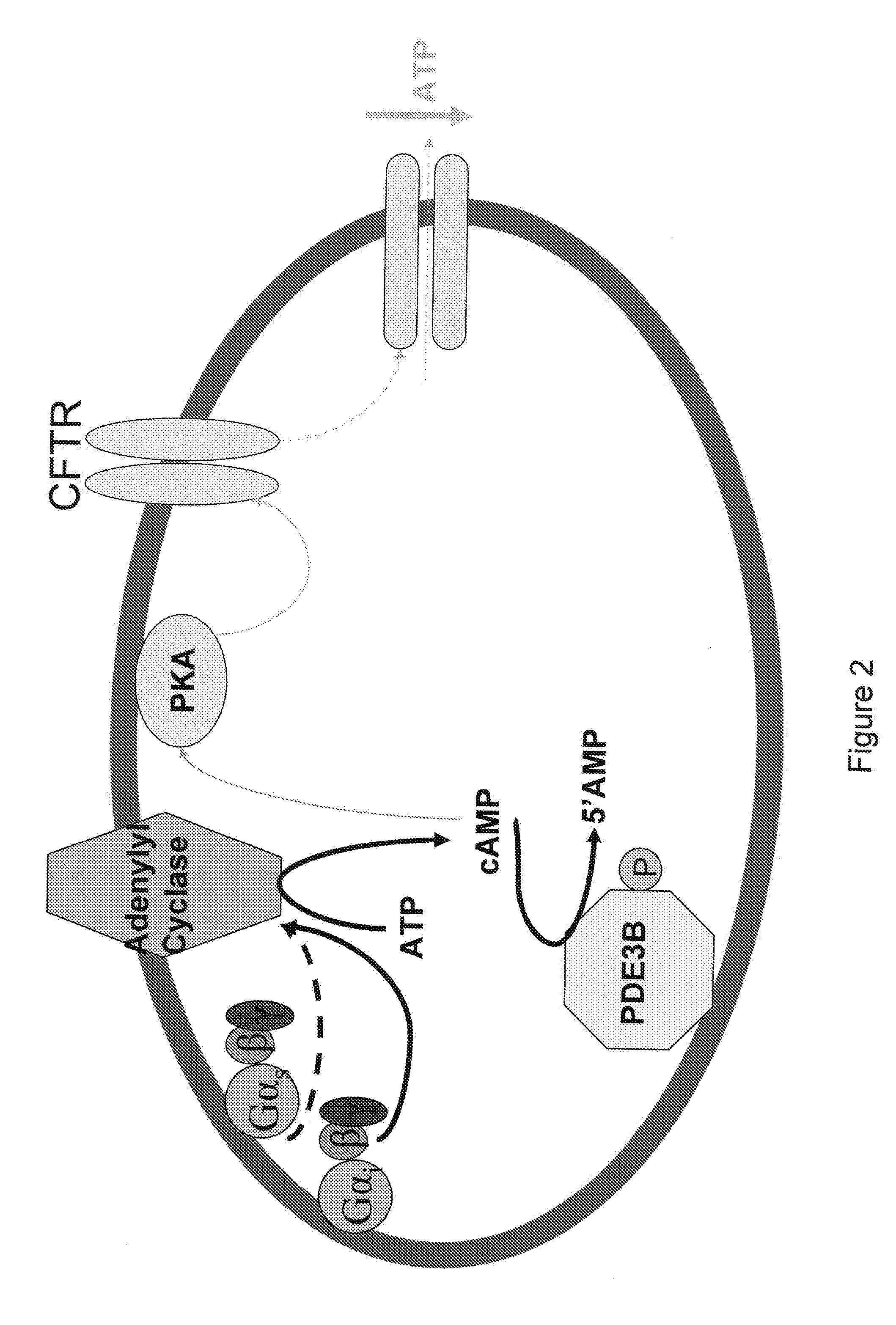
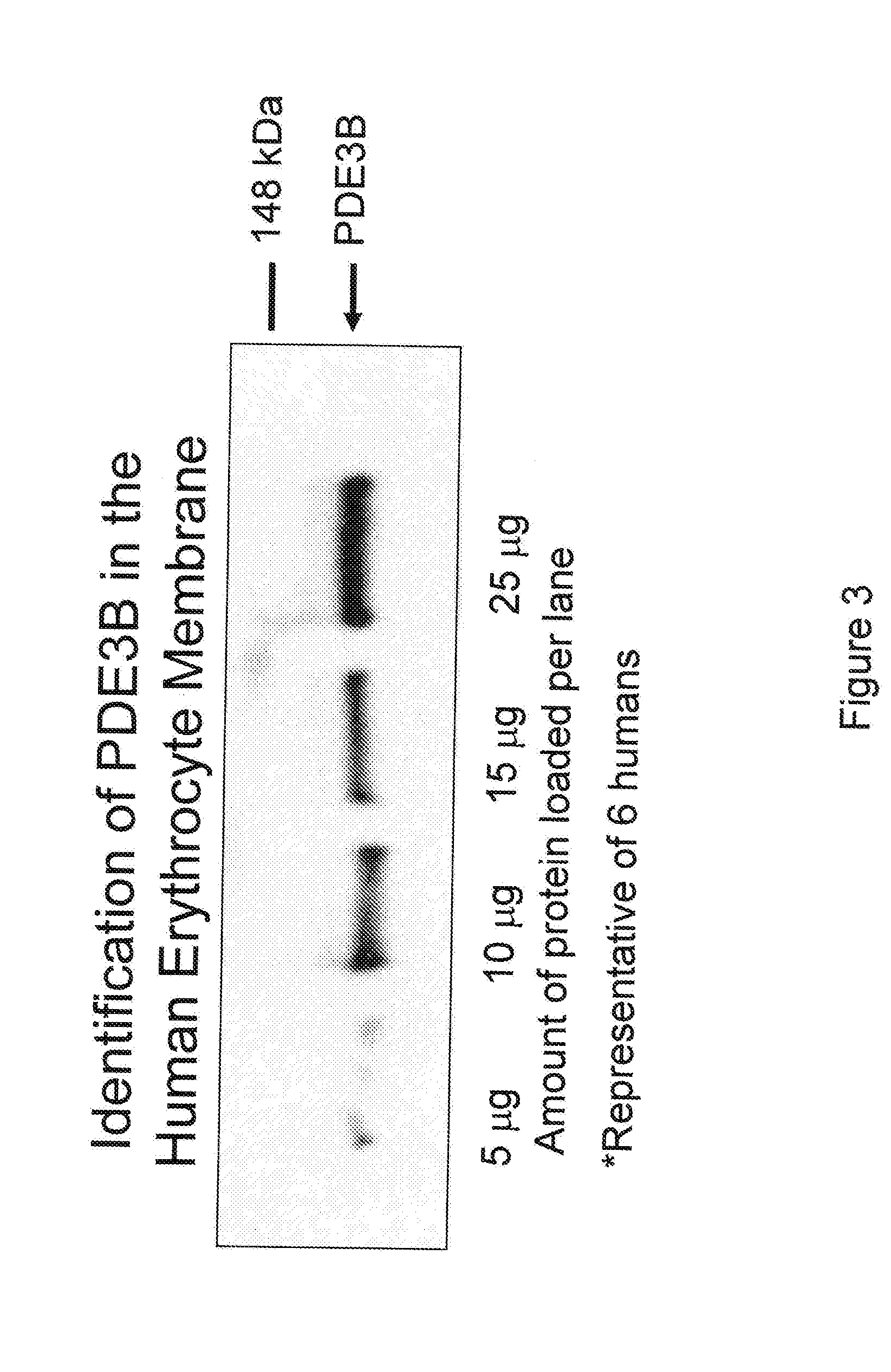
Control of ATP release by red blood cells and therapeutic applications thereof
InactiveUS20070249668A1Increase productionFacilitated releaseBiocideElcosanoid active ingredientsHyperinsulinemiaRed Cell
The invention is based upon the discovery that red blood cells contain phosphodiesterase 3B (PDE3B), and that inhibition of that phosphodiesterase allows for an enhanced accumulation of cAMP and subsequent release of ATP. It was further discovered that RBCs treated with insulin accumulate significantly less cAMP and release significantly less ATP than normal RBCs. Likewise, RBCs of patients suffering from type 2 diabetes (hyperinsulinemia) accumulate significantly less cAMP and release significantly less ATP than normal RBCs. It was further discovered that prostaglandin analogues synergistically work with phosphodiesterase 3B inhibitors to improve or increase cAMP accumulation and ATP release by RBCs. Thus the invention is directed to compositions and methods for improving ATP release by RBCs, via administering PDE3B inhibitor or a combination of PDE3B inhibitor and prostaglandin analogue.
Owner:SAINT LOUIS UNIVERSITY
Transdermal delivery of sildenafil and other phosphodiesterase type 5 inhibitors
InactiveUS20160067252A1BiocideInorganic non-active ingredientsMedicinePhosphodiesterase Type 5 Inhibitors
The present invention generally relates to the transdermal delivery of sildenafil and other phosphodiesterase type 5 inhibitors. While transdermal transport of sildenafil in creams and other topical formulations have been previously described, propylene glycol has not previously been recognized as a transdermal enhancer. However, in some aspects of the present invention, certain concentrations of propylene glycol can have a surprising effect on the transdermal delivery of sildenafil and other phosphodiesterase type 5 inhibitors, e.g., doubling the amount of transdermal delivery in some cases.
Owner:STRATEGIC SCI & TECH
Derivatives of 1-phenyl-2-pyridinyl alkyl alcohols as phosphodiesterase inhibitors
Derivatives of 1-phenyl-2-pyridinyl alkyl alcohols according to formula (I) are useful as inhibitors of the phosphodiesterase 4 (PDE4) enzyme.
Owner:CHIESI FARM SPA
Composition comprising a pulmonary surfactant and a pde5 inhibitor for the treatment of lung diseases
InactiveUS20060148693A1Preventing and reducing onsetReduce severityBiocidePeptide/protein ingredientsPhosphodiesterase 5 inhibitorPde5 inhibition
The invention relates to the combined administration of a pulmonary surfactant and a PDE5 inhibitor for the treatment of a disease in which pulmonary surfactant malfunction and / or phosphodiesterase 5 (PDE5) activity is detrimental.
Owner:TAKEDA GMBH
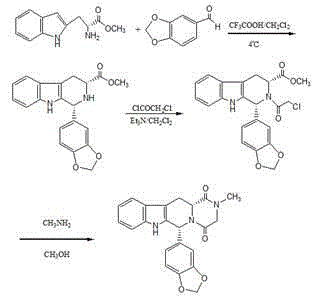
Improved tadalafil preparation method
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
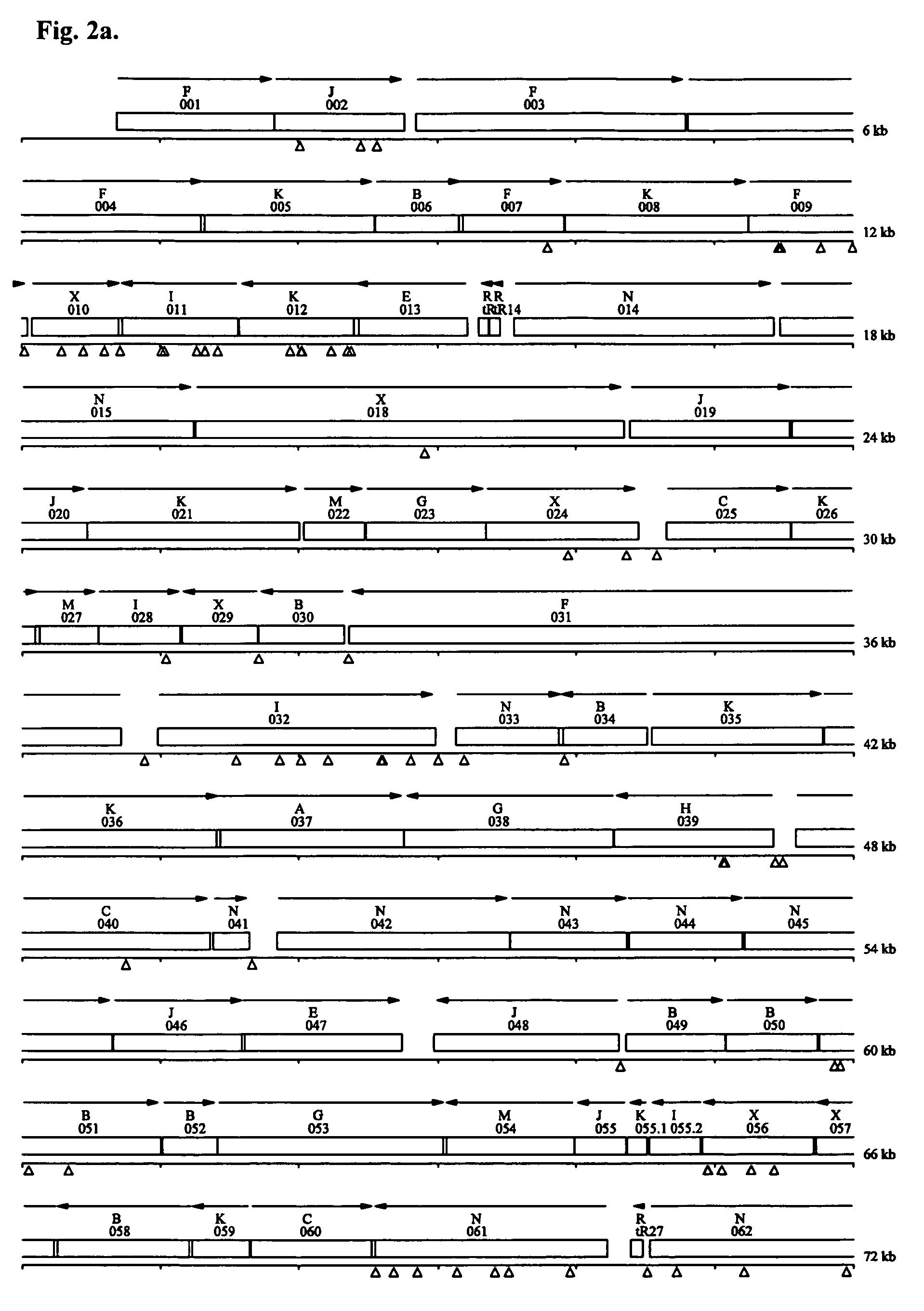
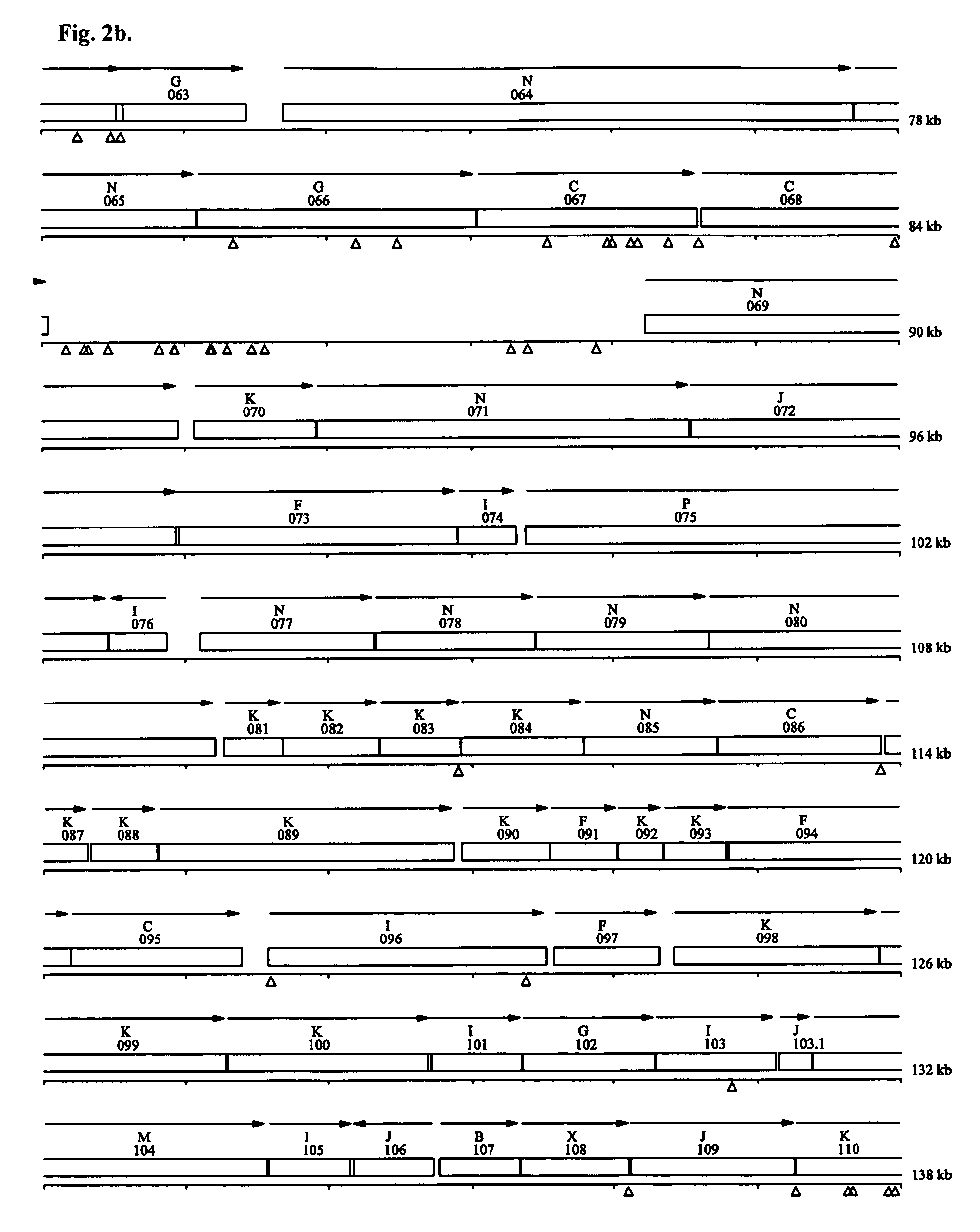
Minimal bacterial genome
The present invention relates, e.g., to a minimal set of protein-coding genes which provides the information required for replication of a free-living organism in a rich bacterial culture medium, wherein (1) the gene set does not comprise the 101 genes listed in Table 2; and / or wherein (2) the gene set comprises the 381 protein-coding genes listed in Table 3 and, optionally, one of more of: a set of three genes encoding ABC transporters for phosphate import (genes MG410, MG411 and MG412; or genes MG289, MG290 and MG291); the lipoprotein-encoding gene MG185 or MG260; and / or the glycerophosphoryl diester phosphodiesterase gene MG293 or MG385.
Owner:SYNTHETIC GENOMICS INC
Method for treatment of multiple sclerosis and related disease states
A method for treatment of multiple sclerosis and related disease states. A histamine H2 mimicking agent is administered in an amount which is effective to stimulate production of a cyclic AMP in the body. A phosphodiesterase inhibitor is administered in conjunction with the histamine H2 mimicking agent to conserve the cyclic AMP which is thus produced. It is believed that the increased cyclic AMP levels serve to maintain the patient's myelin against self degeneration. The histamine H2 mimicking agent may be histamine phosphate and the phosphodiesterase inhibitor may be caffeine. The histamine H2 mimicking agent and the phosphodiesterase inhibitor may be mixed in a gel and administered using a transdermal patch.
Owner:EAD
1-phenyl-2-pyridinyl alkyl alcohol compounds as phosphodiesterase inhibitors
InactiveUS20130012487A1Preventing and/or treating of atopic dermatitisExcellent LPDERespiratorsBiocideAlcoholPhosphodiesterase inhibitor
1-Phenyl-2-pyridinyl alkyl alcohol compounds are effective as inhibitors of the phosphodiesterase 4 (PDE4) enzyme and may be used to prevent and / or treat certain diseases or conditions.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
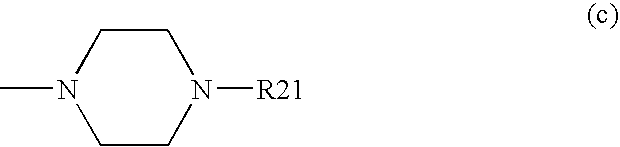
![Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA](https://images-eureka.patsnap.com/patent_img/354b4038-6bc4-4dda-9bb1-fc6c796178da/US08058285-20111115-D00001.png)
![Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA](https://images-eureka.patsnap.com/patent_img/354b4038-6bc4-4dda-9bb1-fc6c796178da/US08058285-20111115-C00001.png)
![Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA Substituted pyrido [3′, 2′: 4, 5] thieno [3, 2-D] pyrimidines and pyrido [3′, 2′: 4, 5] furo [3, 2-D] pyrimidines used as inhibitors of the PDE-4 and/or the release of TNF-ALPHA](https://images-eureka.patsnap.com/patent_img/354b4038-6bc4-4dda-9bb1-fc6c796178da/US08058285-20111115-C00002.png)