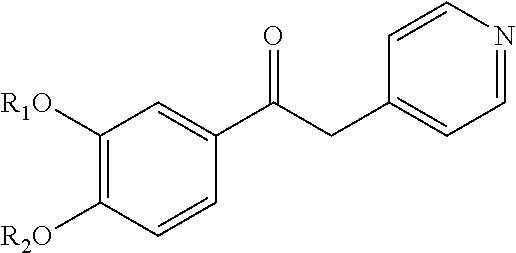
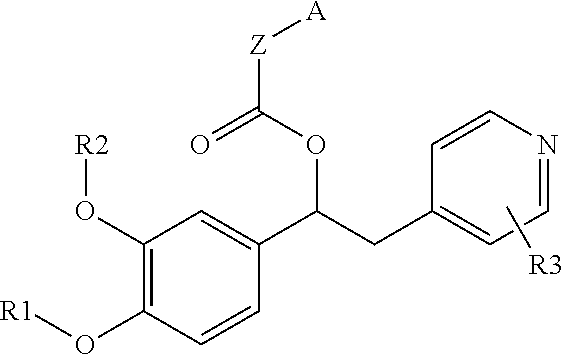
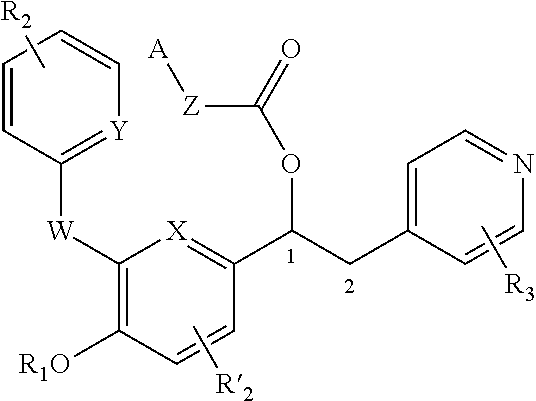
Derivatives of 1-phenyl-2-pyridinyl alkyl alcohols as phosphodiesterase inhibitors
a technology of pyridinyl alkyl alcohol and phosphodiesterase, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problems of limited use of several pde4 inhibitors of the first-generation such as rolipram and piclamilast, and achieve the effect of reducing side effects, appropriate development profile and high affinity for pde4 enzym
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 17
(S)-3,5-dichloro-4-(2-(4-(difluoromethoxy)-3-methoxyphenyl)-2-hydroxyethyl)pyridine 1-oxide 164)
[0488]
Step 1: 4-((S)-2-((S)-2-acetoxy-2-phenylacetoxy)-2-(3-(cyclopropyl-methoxy)-4-(difluoromethoxy)phenyl)ethyl)-3,5-dichloropyridine 1-oxide (161)
[0489]A mixture of (S)-2-acetoxy-2-phenylacetic acid (0.924 g, 4.76 mmol), (S)-3,5-dichloro-4-(2-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)-2-hydroxyethyl)pyridine 1-oxide (1.0 g, 2.380 mmol), EDC (0.684 g, 3.57 mmol), and DMAP (0.436 g, 3.57 mmol) in DCM (150 ml) was stirred at RT for 24 hours. More (S)-2-acetoxy-2-phenylacetic acid (0.350 g, 1.802 mmol), EDC (0.456 g, 2.380 mmol), and DMAP (0.300 g, 2.456 mmol) were added and the stirring was continued for 3 hours to complete conversion. The reaction mixture was washed twice with aqueous 1N HCl and then with aqueous 1M K2CO3; the organic layer was dried over Na2SO4 and evaporated to dryness. The residue was triturated with iPrOH (30 ml) and filtered to afford the desired product (1....
example 18
Synthesis of (S)-3,5-dichloro-4-(2-(3,4-dimethoxyphenyl)-2-hydroxyethyl)pyridine 1-oxide (170)
[0495]
Step 1: Synthesis of 2-(3,5-dichloropyridin-4-yl)-1-(3,4-dimethoxyphenyl)ethanol (166)
[0496]3,5-dichloro-4-methylpyridine (160) (54 g, 331 mmol) was dissolved in dry THF (480 mL) under anargon atmosphere and it was cooled at −78° C. in dry-ice / acetone bath. LHMDS 1N THF solution (331 ml, 331 mmol) was added drop-wise by keeping the temperature at −78°. The mixture was stirred at −78° for 1 hour. After that, a solution of 3,4-dimethoxybenzaldehyde (50 g, 301 mmol) in dry THF (120 ml) was added drop-wise by keeping the temperature at −78° C. When the addition was completed, the mixture was allowed to warm at RT.
[0497]The reaction was poured in ice and water (1 L), and the mixture was stirred until a copious precipitate formed. The solid was filtered, and dissolved in ethyl acetate (500 ml), dried over Na2SO4 and the solvent evaporated under vacuum. The crude was crystallized in CHCl3 / he...
example 36
Synthesis of 3,5-dichloro-4-(2-(2,2-dimethylbenzo[d][1,3]dioxol-5-yl)-2-hydroxyethyl)pyridine 1-oxide (172)
[0506]
Step 1: Synthesis of 2-(3,5-dichloropyridin-4-yl)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)ethanol (171)
[0507]3,5-dichloro-4-methylpyridine (166) (4.37 g, 0.016 mol) was dissolved in dry THF (40 mL) under an argon atmosphere and it was cooled at −78° C. in dry-ice / acetone bath. LHMDS IN THF solution (28 ml, 28 mmol) was added drop-wise by keeping the temperature at −78°. The mixture was stirred at −78° for 1 hour. After that, a solution of 2,3-difluoro-3,4-benzodioxolocarboxaldheyde (5 g, 0.026 mol) in dry THF (10 ml) was added drop-wise by keeping the temperature at −78° C. When the addition was completed, the mixture was allowed to warm at RT. The reaction was poured in ice and water, and the aqueous phase extracted with ethyl acetate (3×). The combined organic phases were dried over Na2SO4 and the solvent evaporated under vacuum. The crude was crystallized in petroleum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com