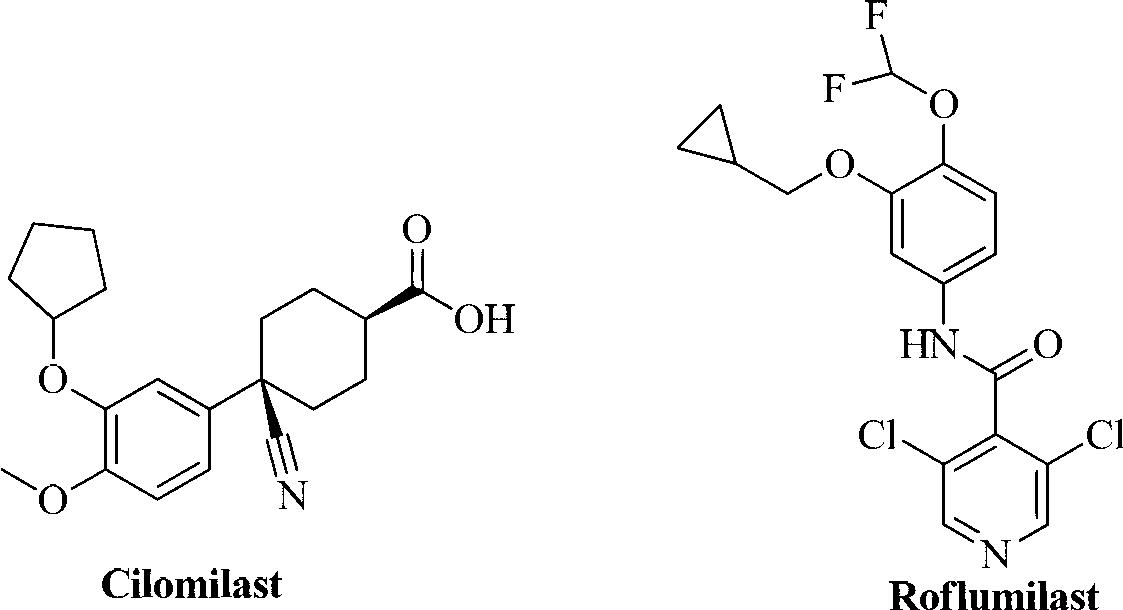
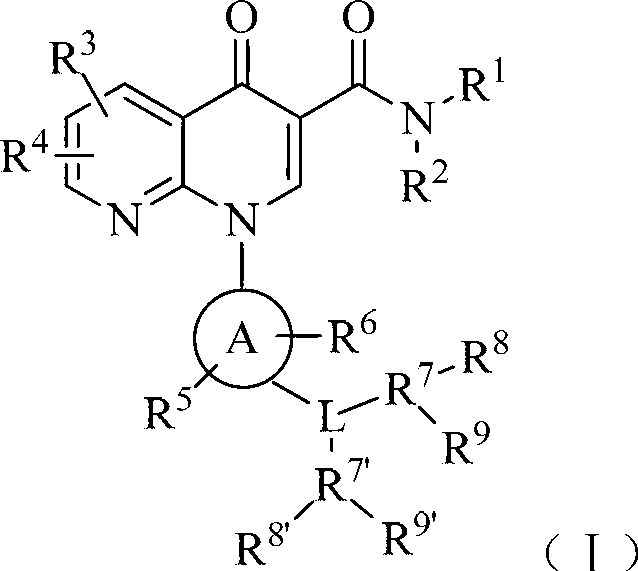
Phosphodiesterase-4 inhibitor
A technology of -ra and q-ra, applied in the field of phosphodiesterase-4 inhibitors, can solve the problems of limiting the dosage, not significantly reducing the acute exacerbation of the disease, and improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
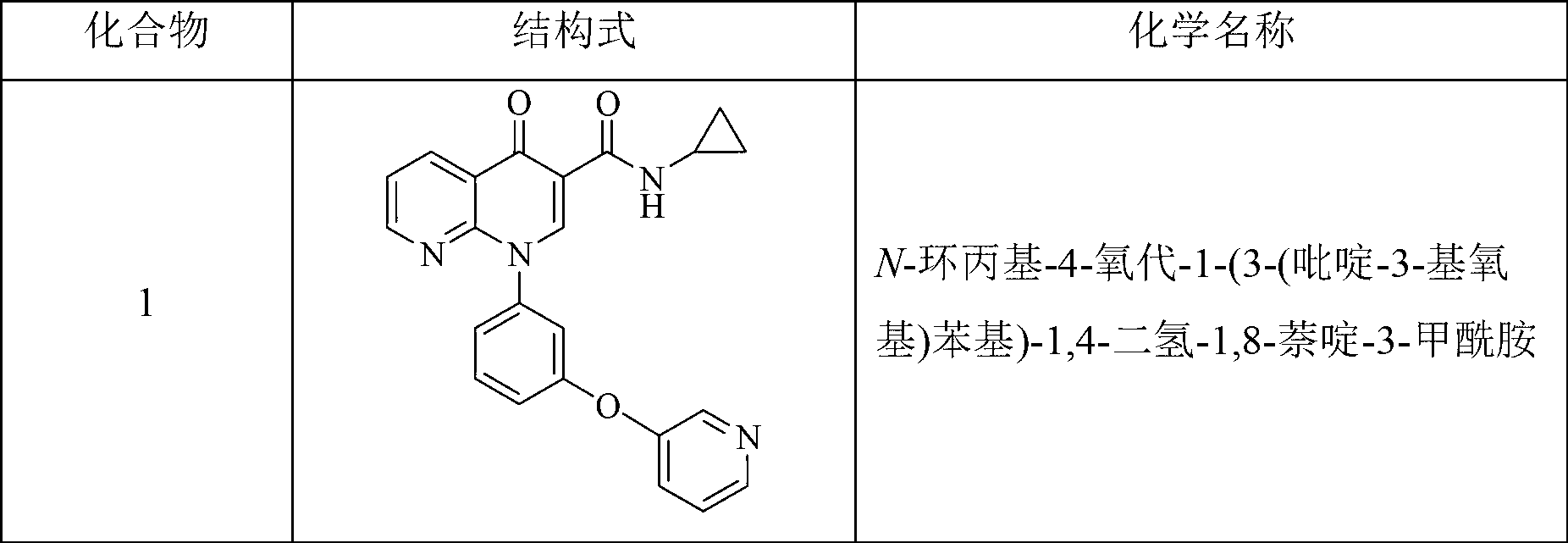
[0143] Example 1 N-cyclopropyl-4-oxo-1-(3-(pyridin-3-yloxy)phenyl)-1,4-dihydro-1,8-naphthyridine-3-carboxamide (compound 1) Preparation
[0144]
[0145] (1) Preparation of 2-chloro-3-pyridyl chloride
[0146]
[0147] Dissolve 2-chloro-3-pyridinecarboxylic acid (15g, 0.095mol) in thionyl chloride, add DMF (N,N-dimethylformamide) (0.5mL), react at 70°C for 3 hours, and depressurize Concentrate, cool and precipitate to obtain the product (13g, yield 78%).
[0148] (2) Preparation of 2-(2-chloronicotinoyl)-3-(dimethylamino)ethyl acrylate
[0149]
[0150] Dissolve 2-chloro-3-pyridine acid chloride (10g, 0.057mol) in toluene (200mL), add TEA (triethylamine) (10.5g, 0.10mol), (E)-3-dimethylaminoacrylic acid Ethyl ester (10g, 0.07mol) was reacted at 90°C for 3 hours, extracted with DCM (dichloromethane) and water, and purified by silica gel column chromatography to obtain the product (13g, yield 81%).
[0151] (3) Preparation of ethyl 1-(3-bromophenyl)-4-oxo-1,4-dih...
Embodiment 2
[0165] Example 2 3-(3-(3-(cyclopropylcarbamoyl)-4-oxo-1,8-naphthyridin-1(4H)-yl)phenoxy)pyridine 1-oxide (C The preparation of nitrogen oxides of compound 1)
[0166]
[0167] The N-cyclopropyl-4-oxo-1-(3-(pyridin-3-yloxy)phenyl)-1,4-dihydro-1,8-naphthyridine- 3-Carboxamide (0.6g, 1.5mmol) and m-chloroperbenzoic acid (m-CPBA) (778mg, 4.5mmol) were dissolved in DCM (200mL) and reacted at 40°C for 3 hours. After the reaction is complete, wash with water, extract with dichloromethane, take the organic phase, dry over anhydrous sodium sulfate, and remove the solvent by rotary evaporation. The residue is separated and purified by silica gel column chromatography (dichloromethane:methanol=50:1) to obtain the product ( 100mg, yield 16%).
[0168] Molecular formula: C 23 h 18 N 4 o 4 Molecular weight: 414.41LC-MS: 415.2 (M+H) +
[0169] 1 H-NMR (400MHz, CDCl 3 )δ: 9.75(s,1H), 9.02(s,1H), 8.79(dd,1H), 8.75(dd,1H), 8.16(s,1H), 8.02(d,1H), 7.60(m,1H ),7.50-7.47(m,1H),7.3...
Embodiment 3
[0170] Example 3 N-cyclopropyl-4-oxo-1-(3-(pyridin-3-ylthio)phenyl)-1,4-dihydro-1,8-naphthyridine-3-carboxamide (compound 2) Preparation
[0171]
[0172] (1) Preparation of 3-nitrothiophenol
[0173]
[0174] Dissolve 1,2-di(3-nitrophenyl)disulfane (20g, 65mmol) in tetrahydrofuran (200mL), add sodium borohydride (8g, 211mmol) dropwise at 0°C, and react at room temperature for 10 After 1 hour, it was quenched with dilute hydrochloric acid, diluted with ethyl acetate, washed with saturated sodium chloride, the organic phase was taken, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation to obtain the product (10 g, yield 99%).
[0175] (2) Preparation of 3-((3-nitrophenyl)thio)pyridine
[0176]
[0177] Under nitrogen, cuprous bromide (758 mg, 5.3 mmol), ethyl 2-oxocyclohexanecarboxylate (1.8 mg, 10.6 mmol) and cesium carbonate (33 g, 0.1 mol) were dissolved in dimethyl sulfoxide (100mL), after stirring at room temperature for 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com