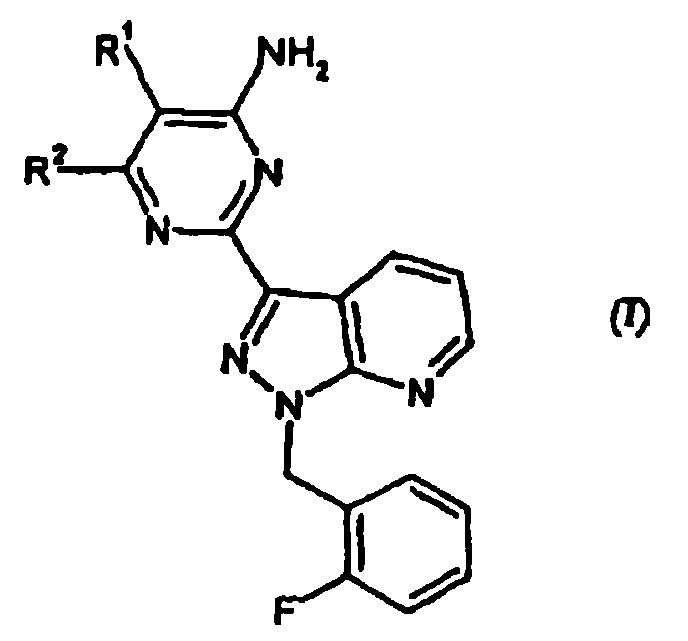
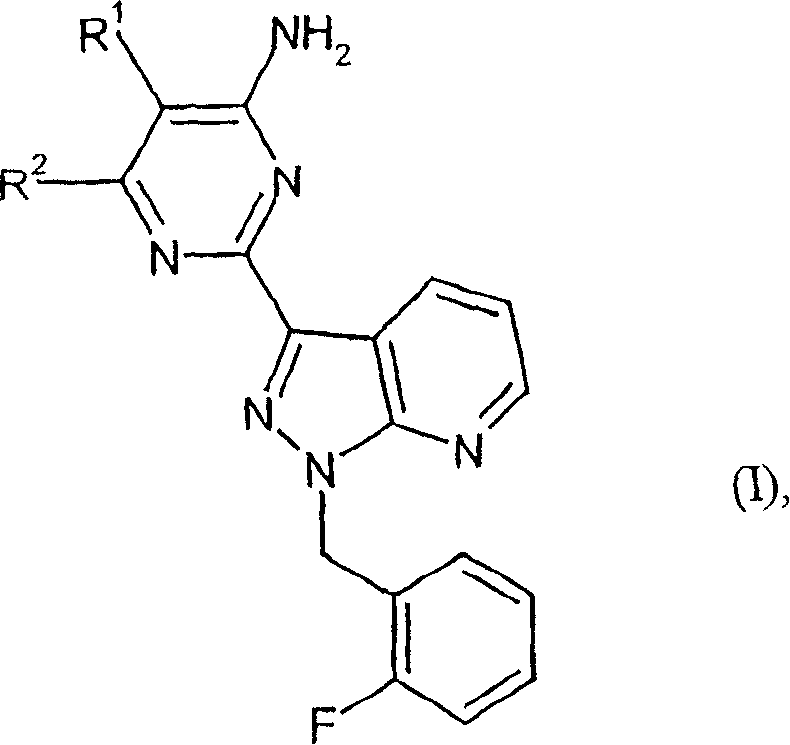
Carbamate-substituted pyrazolopyridines
A compound and hydrate technology, applied in the direction of drug combination, organic chemistry, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
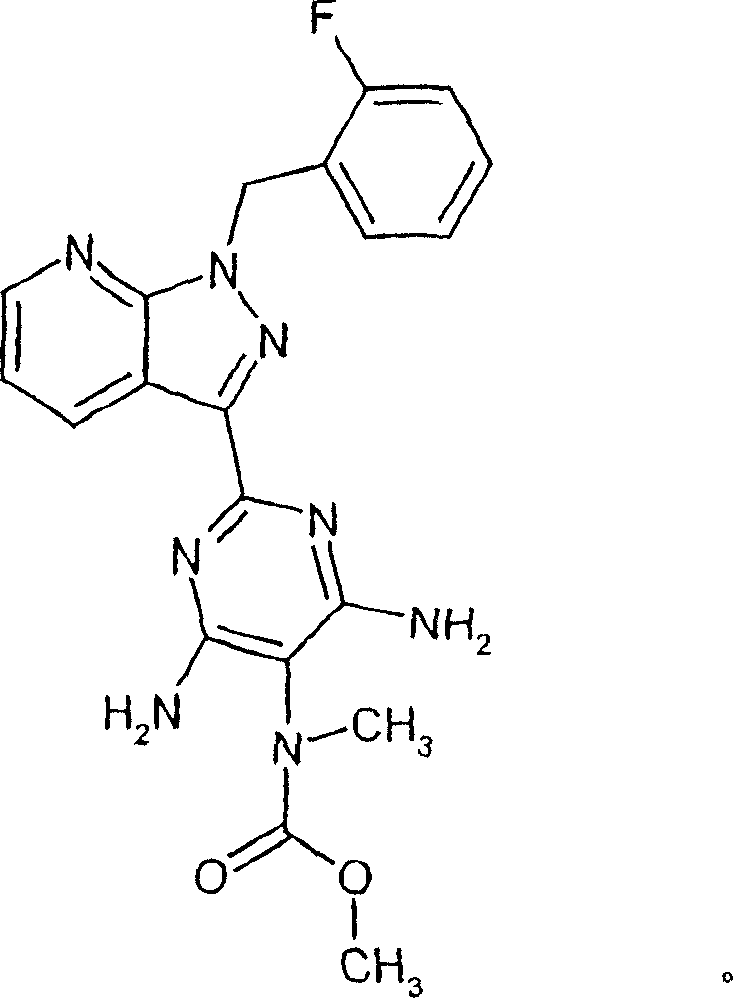
[0225] 4-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl-(methyl)carbamate
[0226]
[0227] Under argon, 0.80 g (2.61 mmol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine from Example 6A, 0.51 g ( 2.86mmol) sodium (E)-2-cyano-2-[(methoxycarbonyl)(methyl)amino]ethenate from Example 10A and 0.53g 0.73ml (5.23mmol) triethylamine were added to 50ml of toluene. The mixture was heated to reflux for 9 hours. It was then recooled to RT, and it was mixed and extracted with dichloromethane and water. The organic phase was dried over magnesium sulfate, filtered and concentrated under vacuum on a rotary evaporator. The residue was mixed with 5 ml of diethyl ether and subsequently crystallized. The crystals were filtered off with suction, dried and purified by preparative RP-HPLC.
[0228] Yield: 20.2 mg (2% of theory)
[0229] LC / MS (Method 2): Rt = 3.01 min
[0230] MS(EI): m / z=408(M+H) +
[0231] 1 H-NMR (300 MHz, DMSO-d 6 ): δ=...
Embodiment 2
[0233] 4,6-Diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinylcarbamate
[0234]
[0235] 107.35 mg (0.31 mmol) of 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-4,5,6 from Example 8A - Pyrimidinetriamine trihydrochloride was added to 5 ml of pyridine and the mixture was cooled to 0°C. To this was added 33.25 mg (0.31 mmol) ethyl chloroformate and the reaction was stirred overnight at RT. Pyridine was evaporated under vacuum with a rotary evaporator and the residue was purified with preparative RP-HPLC.
[0236] Yield: 56.2 mg (43% of theory)
[0237] LC / MS (Method 1): Rt = 2.66 min
[0238] MS(EI): m / z=423(M+H) +
[0239] 1 H-NMR (300MHz, DMSO-d 6 ): δ=1.17-1.33(m, 3H), 3.97-4.14(m, 2H), 5.80(s, 2H), 6.14(broad singlet, 4H), 7.07-7.17(m, 2H), 7.22(t , 1H).7.29-7.40(m, 2H), 7.97(broad singlet, 1H), δ.60(d, 1H), 9.07(d, 1H).
Embodiment 3
[0241] 4,6-Diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinylcarbamate isopropyl ester
[0242]
[0243] Similar to Example 2 with 150 mg (0.43 mmol) of 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]- 4,5,6-Pyrimidinetriamine trihydrochloride, 7.5 ml pyridine and 52.47 mg (0.43 mmol) isopropyl chloroformate. The residue was taken up in a dichloromethane / methanol mixture, filtered and dried.
[0244] Yield: 165 mg (88% of theory)
[0245] LC / MS (Method 1): Rt = 2.84 min
[0246] MS(EI): m / z=437(M+H) +
[0247] 1 H-NMR (300 MHz, DMSO-d 6 ): δ=1.26(d, 6H), 4.82(quintet, 1H), 5.92(s, 2H), 7.07-7.20(m, 2H), 7.25(t, 1H).7.31-7.43(m, 2H ), 7.47-7.57 (m, 1H), 8.16 (broad singlet, 1H), 8.74 (dd, 1H), 8.98 (dd, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com