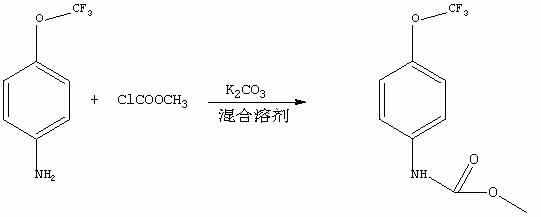
Synthetic method of methyl-4-(trifluoromethoxy) phenyl carbamate
A technology of trifluoromethoxyphenyl and methyl carbamate, which is applied in the preparation and application of fine chemical products, can solve the problems of unsuitability for large-scale production, increase the difficulty of reaction treatment, and limited source of raw materials, and achieve good application The prospect, the preparation method is simple and practical, and the effect of high economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A 150 mL four-necked flask equipped with a reflux and gas absorption device was added to 13.28g (0.075mol) of 4-trifluoromethoxyaniline, 6g of sodium hydroxide (0.15mol), 10ml of tetrahydrofuran and 100ml of water, and mixed well. React for 2h at a temperature of 15-20℃; then, warm to room temperature and stir for 30 minutes. Add 10ml of methyl chloroformate slowly at a rate of 10 seconds / drop, and stir for 6h at room temperature; after the reaction, the temperature is reduced to 0 ℃, the reaction was stirred for 30 minutes; the solid was suction filtered, washed with water, and dried to obtain 16-18g of the target product 4-trifluoromethoxyphenyl-carbamic acid methyl ester, conversion rate: 95%-99% 1 HNMR (400MHz, CDCl 3 ):δ(ppm) 3.67( s, 3H,CH 3 ), 8.0 (t, 1H, NH), 6.75 (d, 2H, ArH). 7.53 (d, 2H, ArH).
Embodiment 2
[0021] A 150 mL four-necked flask equipped with a reflux and gas absorption device was added to 13.28g (0.075 mol) of 4-trifluoromethoxyaniline, 15.9g (0.15 mol) of sodium carbonate, 10ml of dioxane and 100ml of water. After mixing uniformly, react for 2h at a temperature of 10-15℃; then, warm to room temperature and stir for 30 minutes. Add 10ml of methyl chloroformate slowly at a rate of 10 seconds / drop, and stir for 7h at room temperature; after the reaction is complete Cool down to 0℃, stir and react for 30 minutes; the solid is filtered by suction, washed with water, and dried to obtain the target product 4-trifluoromethoxyphenyl-carbamic acid methyl ester 16.5-18g, conversion rate: 95%-99% 1 HNMR (400 MHz, CDCl 3 ): δ (ppm) 3.67 (s, 3H, CH 3 ), 8.0 (t, 1H, NH), 6.75 (d, 2H, ArH). 7.53(d, 2H, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com