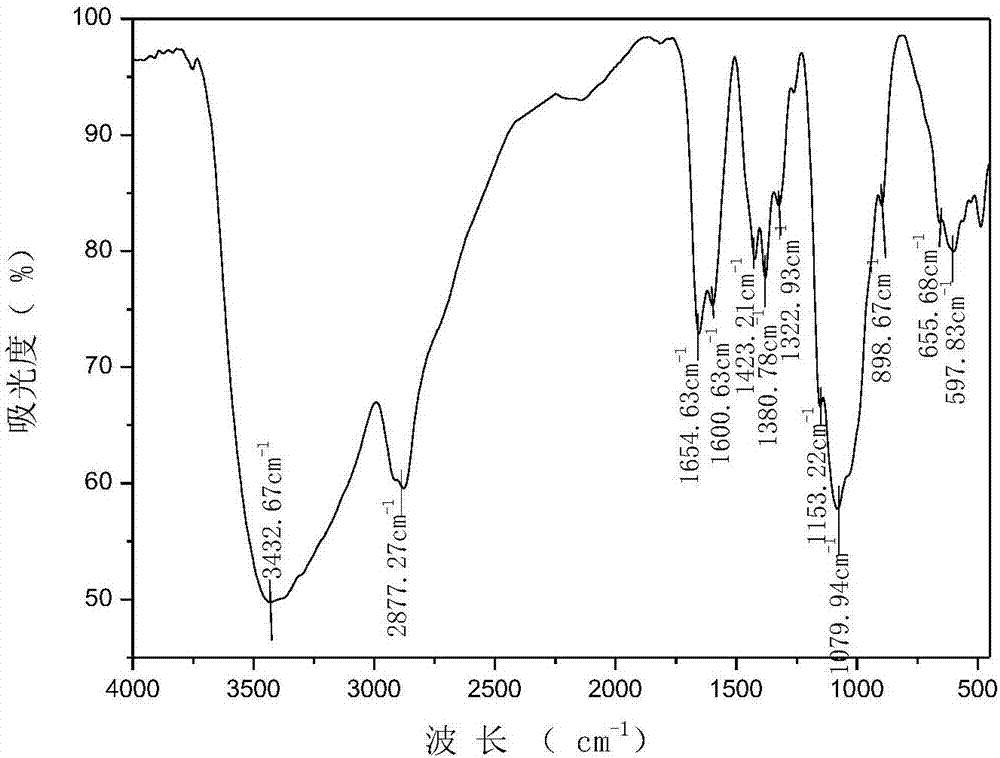
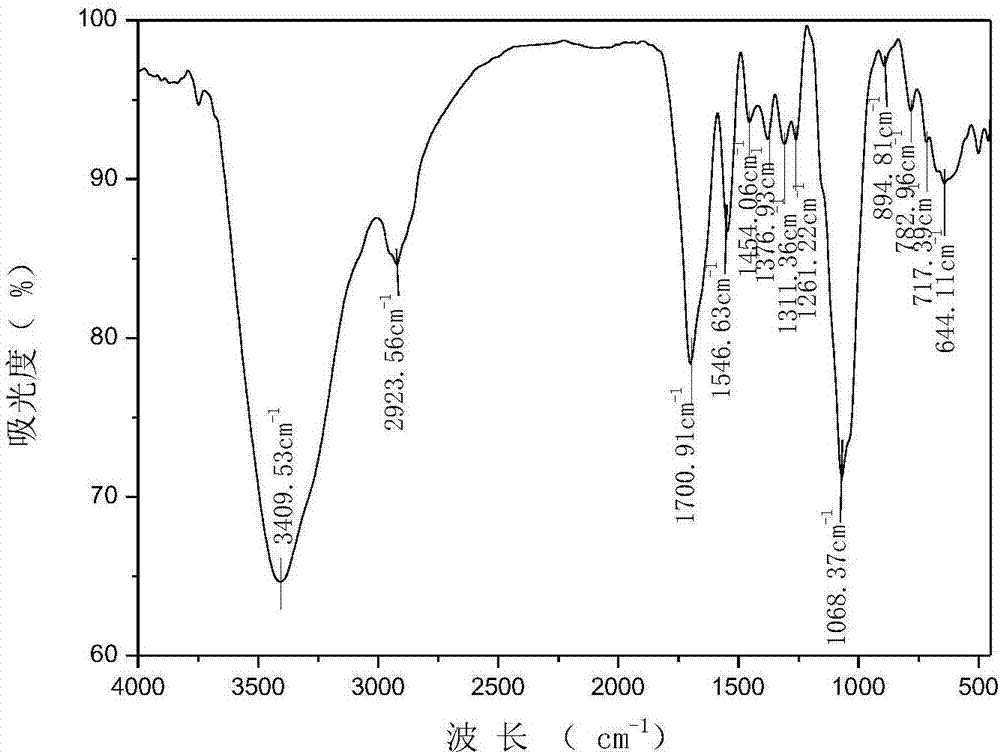
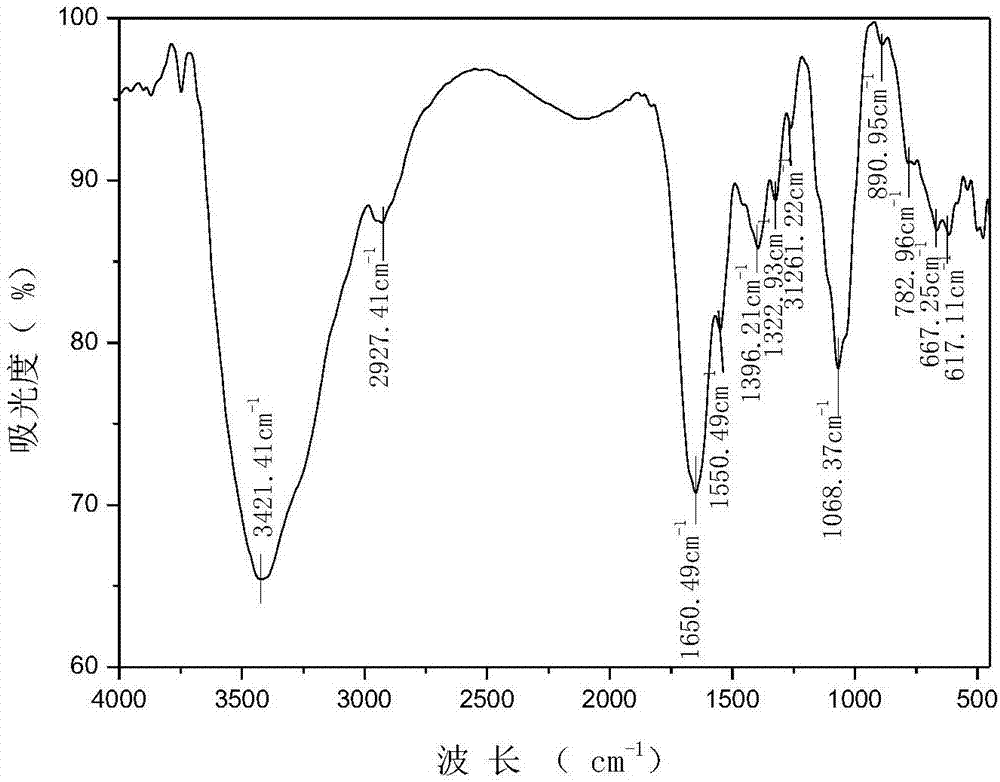
N-pyridylurea chitosan quaternary ammonium salt and preparation method and application thereof
A technology of pyridyl urea-based shell and quaternary ammonium salt, which is applied in the fields of daily chemicals and medicine, can solve the problems of poor water solubility and affect the range of use of chitosan, and achieve low cost, enhanced water solubility and antioxidant activity, and easy promotion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of N-methoxycarbonyl chitosan: Dissolve 1 g of chitosan in 30 mL of distilled water at room temperature, then place it in an ice bath, add 30 mL of methanol when the temperature is lower than 10° C. When the temperature is lower than 5°C, add 2.88mL methyl chloroformate and stir for 6 hours. During this period, the pH value of the solution is controlled by adding triethylamine dropwise to 2-7. After the reaction is completed, precipitate, filter and wash the filter cake with absolute ethanol. Freeze-dry to obtain 1.3 g of the product N-methoxycarbonyl chitosan, which is ready for use.
[0039] (2) N-(2-pyridylureido)-chitosan: get 1gN-methoxycarbonyl chitosan and be dissolved in 20mL mass concentration of 8% lithium chloride in N,N-dimethylacetamide solution , then add 2.58g of 2-aminopyridine, stir and react at 110°C for 12h, precipitate with ethanol directly after the reaction, filter with suction, wash and freeze-dry in vacuum to get N-(2-pyridylureid...
Embodiment 2
[0044](1) Preparation of N-methoxycarbonyl chitosan: Dissolve 1 g of chitosan in 30 mL of distilled water at room temperature, then place it in an ice bath, add 35 mL of methanol when the temperature is lower than 10° C. When the temperature is lower than 5°C, add 3.36mL methyl chloroformate and stir the reaction for 6.5h. During this period, the pH value of the solution is controlled by dropping triethylamine to 2-7. After the reaction is completed, precipitate with absolute ethanol, filter, and wash the filtered The cake was freeze-dried to obtain 1.5 g of the product N-methoxycarbonyl chitosan, which was set aside.
[0045] (2) N-(3-pyridylureido)-chitosan: get 1gN-methoxycarbonyl chitosan and be dissolved in 25mL mass concentration of 8% lithium chloride in N,N-dimethylacetamide solution , then add 3.01g of 3-aminopyridine, stir and react at 110°C for 13h, precipitate with ethanol directly after the reaction, filter with suction, wash and freeze-dry in vacuum to get N-(3-p...
Embodiment 3
[0050] (1) Preparation of N-methoxycarbonyl chitosan: Dissolve 1 g of chitosan in 30 mL of distilled water at room temperature, then place it in an ice bath, add 40 mL of methanol when the temperature is lower than 10° C. When the temperature is lower than 5°C, add 3.84mL methyl chloroformate and stir for 7 hours. During this period, the pH value of the solution is controlled by adding triethylamine dropwise to 2-7. After the reaction is completed, it is precipitated with absolute ethanol and freeze-dried to obtain the product N- Methoxycarbonyl chitosan 1.4g, ready for use.
[0051] (2) N-(4-pyridylureido)-chitosan: get 1g of N-methoxycarbonyl chitosan and be dissolved in 30mL of N,N-dimethylacetamide whose mass concentration is 8% lithium chloride solution, and then add 3.44g of 4-aminopyridine, stirred and reacted at 110°C for 14h, precipitated with ethanol directly after the reaction, and obtained N-(4-pyridylureido) after suction filtration, washing, and vacuum freeze-dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com