Anti-influenza virus compound as well as preparation method and application thereof
A technology for anti-influenza virus and compounds, applied in antiviral agents, organic chemical methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
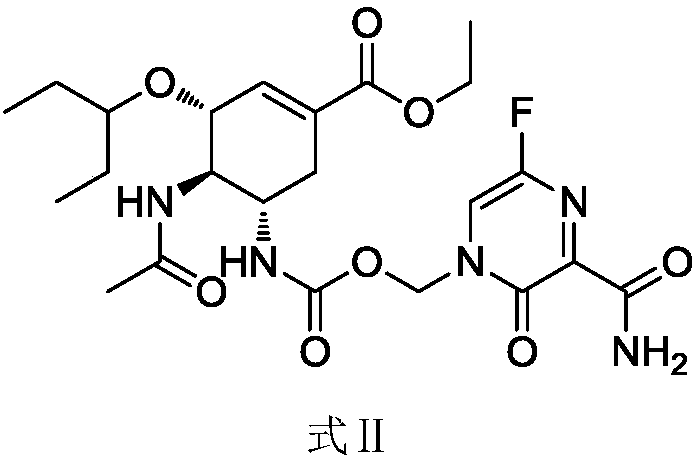
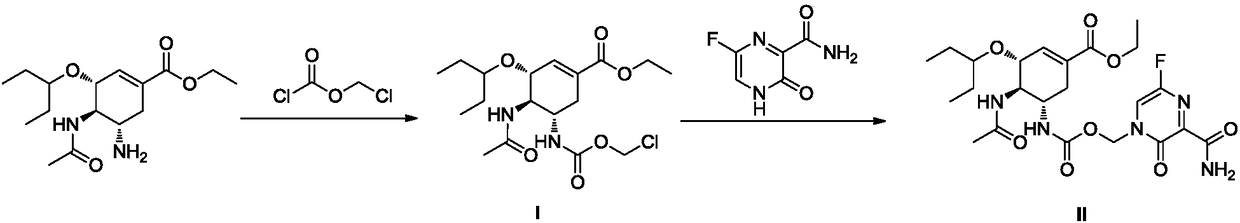
[0033] Embodiment 1: the preparation of anti-influenza virus compound (II)
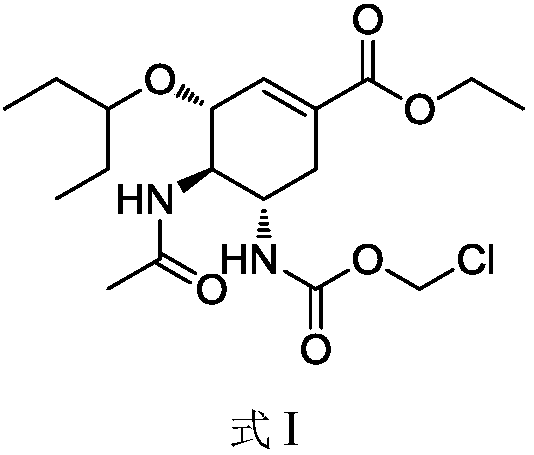
[0034] Step (1) (3R, 4R, 5S)-4-acetamide-5-(chloromethoxycarbonyl)amino-3-(1-propoxyethyl ester)-1-cyclohexane-1-carboxylic acid ethyl ester (I) Preparation
[0035] The reaction equation is:
[0036]
[0037] Acetonitrile (30mL), oseltamivir (3.12g, 10.0mmol), chloromethyl chloroformate (1.94g, 15.0mmol) and N,N-dimethylethylamine (1.10g, 15.0mmol) were successively added to the flask ), stirred at 20°C for 3 hours, after the reaction was completed, the reaction solution was poured into 30 mL of water for extraction, the organic phase was collected, washed with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Filtrate, and evaporate the organic solvent under reduced pressure to obtain 3.45 g of white solid, which is (3R, 4R, 5S)-4-acetamide-5-(chloromethoxycarbonyl)amino-3-(1-propoxyethyl ester) -1-cyclohexane-1-carboxylate ethyl ester (I) crude product, this product...
Embodiment 2
[0042] Embodiment 2: the preparation of anti-influenza virus compound (II)
[0043] Step (1) (3R, 4R, 5S)-4-acetamide-5-(chloromethoxycarbonyl)amino-3-(1-propoxyethyl ester)-1-cyclohexane-1-carboxylic acid ethyl ester (I) Preparation
[0044] The reaction equation is:
[0045]
[0046] Tetrahydrofuran (30ml), oseltamivir (3.12g, 10.0mmol), chloromethyl chloroformate (1.29g, 10.0mmol) and N,N-diisopropylethylamine (1.94g, 15.0 mmol), stirred at room temperature for 5 hours, the reaction was completed, the reaction solution was poured into 30ml of water for extraction, the organic phase was collected, washed with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Filtrate, and evaporate the organic solvent under reduced pressure to obtain 3.82 g of white solid, which is (3R, 4R, 5S)-4-acetamide-5-(chloromethoxycarbonyl)amino-3-(1-propoxyethyl ester) - 1-Cyclohexane-1-carboxylate ethyl ester (I) crude product, this product is directly used in the n...
Embodiment 3
[0051] Example 3: In vitro antiviral activity test of compound (II) containing oseltamivir and favipiravir structures
[0052] (1) Test method
[0053] Chicken embryo fibroblasts were inoculated in 96-well culture plates, and the culture solution containing influenza virus and the compound to be tested was added, and the cells were incubated at 37°C, 5% CO 2 After culturing in medium for 48 hours, 10 μL of cck-8 reagent and 90 μL of DMEM matrix containing 1% FBS were added, and the culture was continued for 90 minutes at 37° C. to observe cytopathic changes (CPE) to determine the inhibitory effect of each compound on influenza virus. Oseltamivir carboxylate was used as a positive control.
[0054] (2) Test results
[0055] Table 1 Compound II in vitro antiviral activity test results
[0056]
[0057] It can be seen from the above table that Compound II has different degrees of inhibitory effects on H5N2, H5N6, and H5N8, and has better antiviral activity on H5N2 in vitro....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com