Preparation process of amino acid N-carboxylic acid anhydride
A preparation process and amino acid technology, applied in the field of chemical synthesis and production, can solve problems such as hidden safety and environmental protection risks, and the synthesis method has not achieved major breakthroughs, and achieves low environmental pollution, simple and convenient post-processing operations, and low energy consumption in the production process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
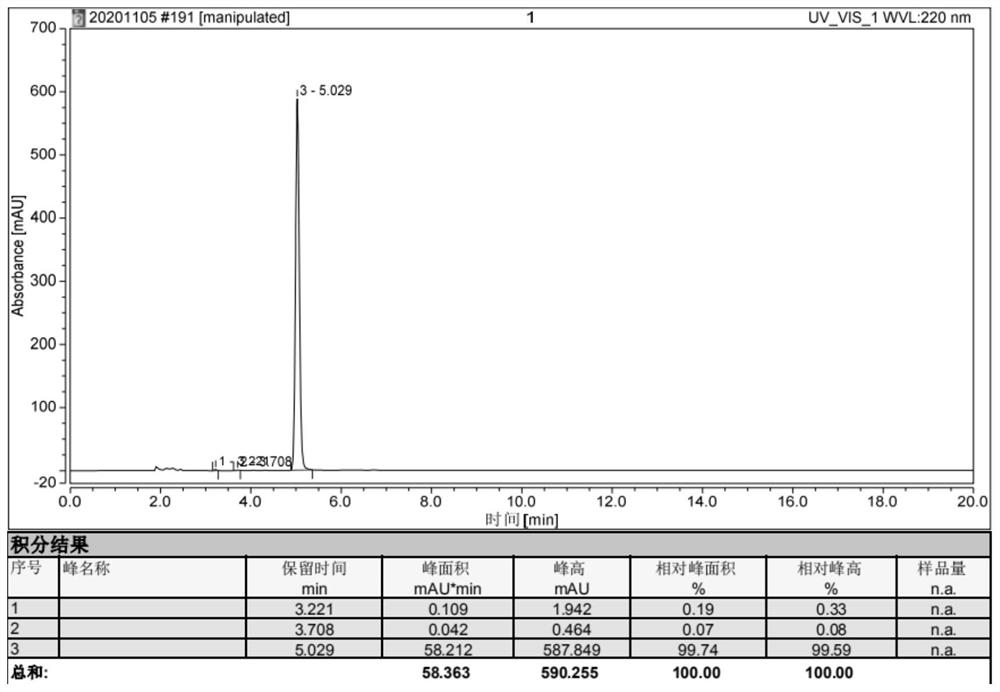
[0050] The synthesis of embodiment 1,4-isopropyl-2,5-oxazolidinedione:
[0051] The reaction process is as follows:
[0052] STEP1: Synthesis of N-(methoxycarbonyl)-L-valine
[0053] Take 1.17Kg (10mol) of L-valine, 5L of 2mol / L sodium hydroxide solution, and 1.06Kg (10mol) of sodium carbonate into a 20L reaction kettle, start stirring, and after the L-valine is completely dissolved, dissolve the solution The temperature dropped to -10°C, and 5L of 1,4-dioxane solution containing 1.89Kg (20mol) of methyl chloroformate was added dropwise. The temperature of the solution was kept below 20°C during the dropwise addition, and the reaction was carried out at 25°C for 8 hours after dropping. After the reaction is complete, the reaction solution is extracted with 2.5 L of ethyl acetate, the organic phase is discarded, the water phase is cooled to below 10°C, concentrated hydrochloric acid is added dropwise until the pH is 2, and then stirred at 10°C for 30 minutes, a large amount ...
Embodiment 2
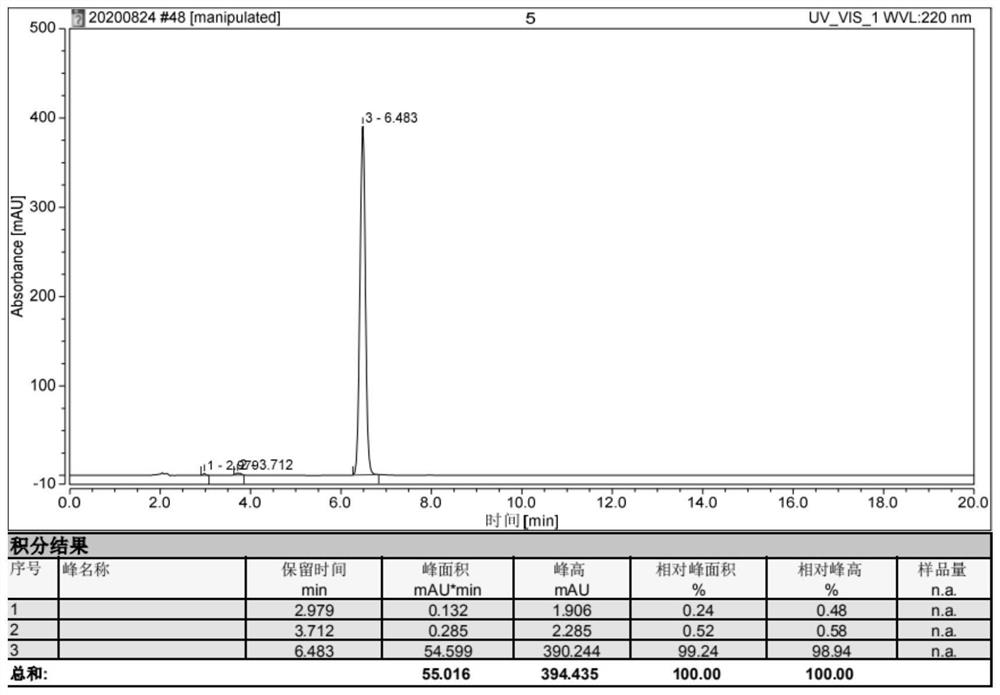
[0056] Embodiment 2, the synthesis of 4-((1H-indol-3-yl)methyl)oxazolidine-2,5-dione:
[0057] The reaction process is as follows:
[0058]
[0059] STEP1: Synthesis of N-(methoxycarbonyl)-L-tryptophan
[0060] Take 2.04Kg (10mol) of L-tryptophan, 5L of 10% potassium hydroxide solution, and 1.38Kg (10mol) of potassium carbonate into a 20L reaction kettle, start stirring, and after the L-tryptophan is completely dissolved, lower the temperature of the solution Drop below -10°C, add dropwise 5L of a tetrahydrofuran solution containing 1.89Kg (20mol) of methyl chloroformate, keep the temperature of the solution below 20°C during the dropwise addition, and react at 40°C for 12 hours after dropping. After the reaction is complete, the reaction solution is extracted with 2.5 L of ethyl acetate, the organic phase is discarded, the water phase is cooled to below 10°C, concentrated hydrochloric acid is added dropwise until the pH is 3, and then stirred at 10°C for 30 minutes, a lar...
Embodiment 3
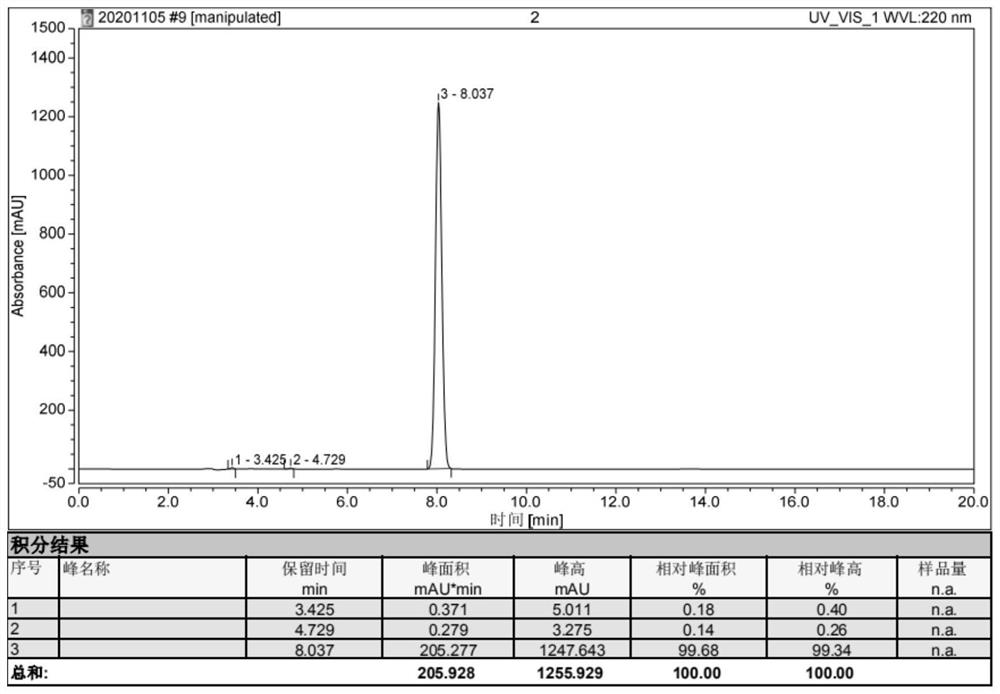
[0063] Embodiment 3, the synthesis of (2,5-dioxazolidin-4-yl) methyl methyl carbonate:
[0064] The reaction process is as follows:
[0065]
[0066] STEP1: Synthesis of N,O-bis(methoxycarbonyl)-L-serine
[0067] Take 1.05Kg (10mol) of L-serine, 10L of 2mol / L sodium hydroxide solution, and 2.12Kg (20mol) of sodium carbonate into a 20L reaction kettle, start stirring, and after the L-serine is completely dissolved, lower the temperature of the solution to - Below 10°C, add dropwise 5L of tetrahydrofuran solution containing 2.84Kg (30mol) of methyl chloroformate, keep the temperature of the solution below 20°C during the dropping process, and react at 60°C for 8h after the drop is complete. After the reaction was completed, the reaction solution was extracted with 3 L of ethyl acetate, the organic phase was discarded, the water phase was cooled to below 10°C, concentrated hydrochloric acid was added dropwise until the pH was 2, and then stirred at 10°C for 30 minutes, a larg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com