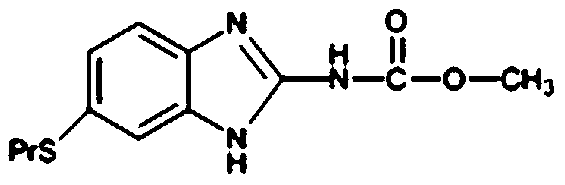
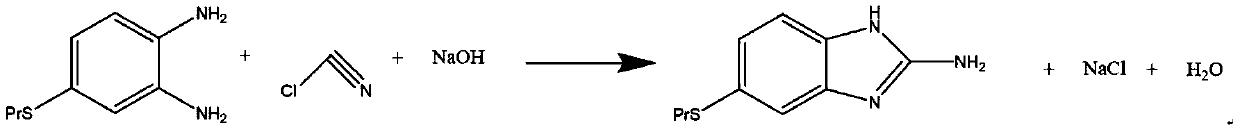
Preparation method of albendazole
A technology of albendazole and benzo, which is applied in the field of preparation of veterinary medicine and pharmaceutical raw material albendazole, which can solve problems such as unstable product yield and quality, high pressure of three wastes treatment, and difficulty in grasping cyclization conditions, etc. , to achieve strong reactivity, increase yield, and solve the dual contradictions of economy and environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take a 250mL four-neck flask, add 20.05g of 4-propylthio-o-phenylenediamine and 60.15g of methanol, and stir to dissolve. Using a freezer, the temperature was lowered to 3° C., and a peristaltic pump was used to slowly add 8.13 g of cyanogen chloride dropwise at a speed of 0.14 g / min for 1 hour. During the process, the temperature was maintained at 3° C., and 10% sodium hydroxide solution was added dropwise to adjust the pH=4. After the dropwise addition, the temperature was raised to 45° C. and kept for 1 h. Atmospheric distillation was then carried out to recover methanol, the solid-liquid mixed aqueous solution was cooled to 20°C, and the solid part was obtained by filtering through filter paper, and dried in a vacuum oven to obtain 21.71g of 6-propylthio-2-amine-1-hydro-benzo[ d] imidazole. The calculated yield is 95.21%.
[0028] Dissolve all 6-propylthio-2-amine-1-hydrogen-benzo[d]imidazole in 100g of methanol, cool down to 8°C, and slowly add 10.90g of chlorin...
Embodiment 2
[0030] Take a 250mL four-neck flask, add 20.12g of 4-propylthio-o-phenylenediamine and 60.36g of methanol, and stir to dissolve. Using a refrigerator, the temperature was lowered to 3° C., and a peristaltic pump was used to slowly add 7.48 g of cyanogen chloride dropwise at a speed of 0.12 g / min for 1 hour. During the process, the temperature was maintained at 3° C., and 10% sodium hydroxide solution was added dropwise to adjust the pH=5. After the dropwise addition, the temperature was raised to 45° C. and kept for 1 h. Atmospheric distillation is then carried out to recover methanol, the solid-liquid mixed aqueous solution is cooled to 20°C, and the solid part is obtained by filtering through filter paper, and after drying in a vacuum oven, 21.65 g of 6-propylthio-2-amine-1-hydro-benzo[ d] imidazole. The calculated yield is 94.62%.
[0031] Dissolve all 6-propylthio-2-amine-1-hydrogen-benzo[d]imidazole in 100g of methanol, cool down to 8°C, slowly add 10.87g of chlorine d...
Embodiment 3
[0033] Take a 250mL four-neck flask, add 20.36g of 4-propylthio-o-phenylenediamine and 61.08g of methanol, and stir to dissolve. Use a freezer to lower the temperature to 3°C, and slowly add 6.88g of cyanogen chloride dropwise at a rate of 0.11g / min with a peristaltic pump for 1 hour. During the process, the temperature was maintained at 3° C., and 10% sodium hydroxide solution was added dropwise to adjust the pH=4. After the dropwise addition, the temperature was raised to 45° C. and kept for 1 h. Atmospheric pressure distillation is then carried out to recover methanol, the solid-liquid mixed aqueous solution is cooled to 20°C, and the solid part is obtained by filtering through filter paper. After drying in a vacuum oven, 21.45 g of 6-propylthio-2-amine-1-hydro-benzo[ d] imidazole. The calculated yield is 92.64%.
[0034] Dissolve all 6-propylthio-2-amine-1-hydrogen-benzo[d]imidazole in 100g of methanol, cool down to 8°C, slowly add 10.77g of chlorine dropwise at a speed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com