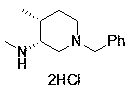
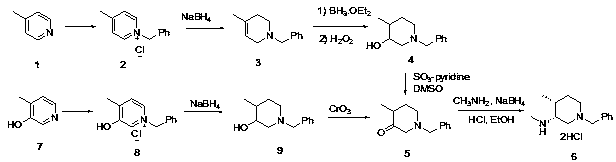
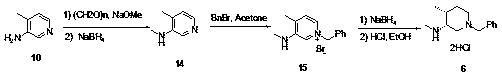Novel method for synthesizing cis-1-benzyl-3-methylamino-4-methyl-piperidine
A kind of methylamino, benzyl technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0030] Preparation Example 1: Synthesis of Compound 6 and Preparation of Intermediates
[0031] Add 4-amino-3-picoline 10 (10 g, 92.4 mmol) to a 250 ml reaction flask, add methanol (100 g), start stirring, and when the temperature is lower than 30 degrees, add sodium methoxide (7.48 g, 138.6 mmol ), add paraformaldehyde (5.54 g, 184 mmol), and complete the addition. The reaction solution was stirred overnight, and the HPLC detected that the raw material was less than 3%, and it was judged that the first-stage reaction was completed. At a temperature lower than 30°C, sodium borohydride (7.0 g, 184.8 mmol) was added in batches and stirred for 5-8 hours. The HPLC detection of raw materials was less than 3%, and the reaction was judged to be complete. The reaction solution was concentrated under reduced pressure to half, and the reaction was quenched with 3M hydrochloric acid at a temperature lower than 30 degrees, extracted twice with dichloromethane, the organic phases were com...
preparation Embodiment 2
[0034] Preparation Example 2: Synthesis of Compound 6
[0035] Add 4-amino-3-picoline 10 (10 g, 92.4 mmol) to a 250 ml reaction flask, add methanol (100 g), start stirring, and when the temperature is lower than 30 degrees, add sodium methoxide (11.22 g, 207.9 mmol ), add paraformaldehyde (2.77 g, 92 mmol), and complete the addition. The reaction solution was stirred overnight, and the HPLC detected that the raw material was less than 3%, and it was judged that the first-stage reaction was completed. At a temperature lower than 30°C, sodium borohydride (3.5g, 92.4mmol) was added in batches and stirred for 5-8 hours. The HPLC detection of raw materials was less than 3%, and the reaction was judged to be complete. The reaction solution was concentrated under reduced pressure to half, and the reaction was quenched with 3M hydrochloric acid at a temperature lower than 30 degrees, extracted twice with dichloromethane, the organic phases were combined, and concentrated under reduce...
preparation Embodiment 3
[0036] Preparation Example 3: Synthesis of Compound 6
[0037] Add 4-amino-3-picoline 10 (10 g, 92.4 mmol) to a 250 ml reaction flask, add methanol (100 g), start stirring, and when the temperature is lower than 30 degrees, add sodium methoxide (4.99 g, 92.4 mmol ), add paraformaldehyde (8.31 g, 277mmol), and complete the addition. The reaction solution was stirred overnight, and the HPLC detected that the raw material was less than 3%, and it was judged that the first-stage reaction was completed. At a temperature lower than 30°C, sodium borohydride (3.5 g, 92.4 mmol) was added in batches and stirred for 5-8 hours. HPLC detected that the raw material was less than 3%, and the reaction was judged to be complete. The reaction solution was concentrated under reduced pressure to half, and the reaction was quenched with 3M hydrochloric acid at a temperature lower than 30 degrees, extracted twice with dichloromethane, the organic phases were combined, and concentrated under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com