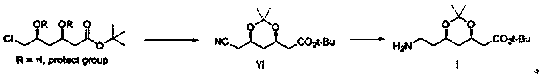
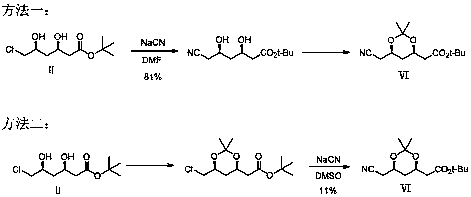
Preparation method of atorvastatin calcium intermediate
A technology of atorvastatin calcium and its intermediates, which is applied in the field of medicine and chemical industry, can solve the problems of harm to human body, difficulty in purchasing, increasing the cost of atorvastatin calcium preparation, etc., and achieve the effect of reagent safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] At 0°C, metal lithium (0.18g) was dissolved in 40mL of anhydrous ether, and 10ml of ether solution of compound II (2.79g, 0.01mol) was slowly added dropwise to it, and stirred at 0°C. During the reaction, a white precipitate was produced. After reacting until the lithium reaction is complete, let it stand still, and take the supernatant containing compound III for the next reaction.
[0046] Methyl chloroformate (1.09g, 0.012mol) was dissolved in 10ml of anhydrous ether, and the supernatant containing compound III obtained in the previous step (diethyl ether solution of compound III) was added dropwise thereto at -20°C , After the dropwise addition is completed, warm up to room temperature (15~25°C), and stir until the reaction is completed. Then lower the temperature to 0°C, and add 6.5mL saturated ammonium chloride solution to the reacted solution, then add 2.2ml hydrochloric acid with a mass percentage of 10%, keep the organic layer, wash with 5% sodium bicarbonate, ...
Embodiment 2
[0051] At 0°C, metal lithium (0.18g) was dissolved in 40mL of anhydrous tetrahydrofuran, and 10ml of compound II (2.79g, 0.01mol) in tetrahydrofuran was slowly added dropwise, and stirred at 0°C. During the reaction, a white precipitate was produced. After reacting until the lithium reaction is complete, let it stand still, and take the supernatant containing compound III for the next reaction.
[0052] Methyl chloroformate (1.09 g, 0.012 mol) was dissolved in 10 mL of anhydrous tetrahydrofuran. At -20°C, add dropwise the supernatant containing compound III obtained in the previous step (the tetrahydrofuran solution of compound III), rise to room temperature (15~25°C), stir, and after the reaction is completed, Add 6.5mL of saturated ammonium chloride solution, then add 2.2ml of 10% hydrochloric acid, retain the organic layer, wash with 5% sodium bicarbonate, remove THF by rotary evaporation, and obtain 2.05g of compound IV (purity: 92%, yield: 68%) .
[0053] Compound IV (6...
Embodiment 3
[0057] At 0°C, metal lithium (0.18g) was dissolved in 40mL of anhydrous ether, and 10ml of ether solution of compound II (2.79g, 0.01mol) was slowly added dropwise to it, and stirred at 0°C. During the reaction, a white precipitate was produced. After reacting until the lithium reaction is complete, let it stand still, and take the supernatant containing compound III for the next reaction.
[0058] Methyl chloroformate (1.09 g, 0.012 mol) was dissolved in 10 mL of anhydrous ether. Add the supernatant containing compound III (diethyl ether solution of compound III) obtained in the previous step dropwise to it at -20°C, rise to room temperature (15~25°C), stir, and after the reaction is completed, Saturated ammonium chloride solution was added, followed by 2.2ml of 10% hydrochloric acid, the organic layer was retained, washed with 5% sodium bicarbonate, and ether was removed by rotary evaporation to obtain 2.11g of compound IV (93% purity, 70% yield).
[0059] In compound IV (6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com