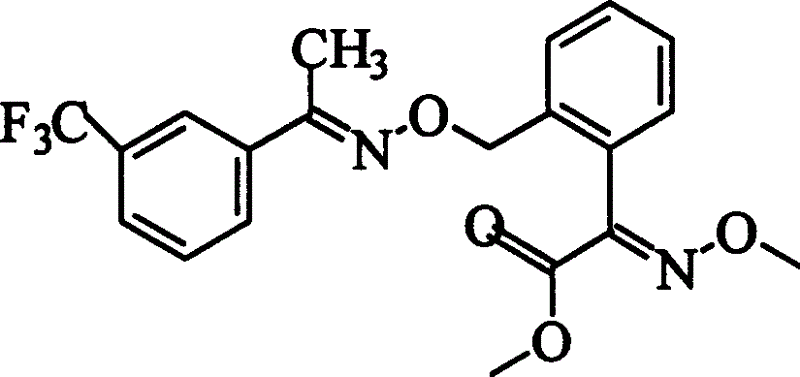
Preparation process of oxime strain ester
A technology of trifloxystrobin and ketoxime, which is applied in the field of preparation of organic compounds, can solve the problem of not providing a synthesis process, etc., and achieves the effects of mild operating conditions, reduction of waste water discharge, and avoidance of esterification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of 2-(2'-methylphenyl)-2-oxoacetic acid
[0020] Add 2ml (0.015mol) of o-methyl acetophenone, 6g of sodium hydroxide, and 200ml of water into a 500ml round bottom flask, and add 7g of potassium permanganate into the flask in batches under stirring in an ice-water bath. When the last batch of potassium permanganate is added, the color in the reaction system remains unchanged for 1 hour. After the reaction is completed, add sodium bisulfite solution dropwise to make the purple color of the mixed solution fade away, and remove the manganese dioxide solid by suction filtration. Ether extraction removed unreacted starting material. The aqueous phase was acidified to pH=4 with concentrated hydrochloric acid, a white solid appeared, extracted with 2 x 50ml ether, and the white solid was dissolved in ether. The collected organic phase was dried with anhydrous magnesium sulfate, and diethyl ether was evaporated to obtain 2 g of yellow 2-(2'-methylphenyl)-2-oxoacetic a...
Embodiment 2
[0041] Other steps carry out embodiment 1 repeatedly in the same steps, but wherein the synthesis of 2-(2'-bromomethylphenyl)-2-oxomethyl acetate of the 3rd step is carried out according to the following steps: 1g (0.006mol) -(2'-methylphenyl)-2-oxoacetic acid methyl ester was dissolved in 20ml of carbon tetrachloride and placed in a 100ml three-necked flask, and 1.5g (0.012mol) of NBS (N-bromosuccinimide) was added and 0.2gBPO (benzoyl peroxide), irradiated with a 250W mercury lamp. During the reaction, TLC (ethyl acetate:petroleum ether=1:8) was used to track the progress of the reaction, and TLC showed that the raw material point basically disappeared to end the reaction. The solution was washed once with 50ml water, saturated sodium bicarbonate (NaHCO 3 ) solution for three washes. The organic phase was dried over anhydrous magnesium sulfate, and the solvent was distilled off to obtain a yellow-brown product. Purified by silica gel column chromatography (ethyl acetate:p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com