Method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate
A bisoxopiperazine and formate technology, applied in the field of intermediate preparation, can solve the problems of high price, high product cost, cumbersome post-processing route, etc., and achieve simple process operation, high atom utilization rate, and equipment utilization high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
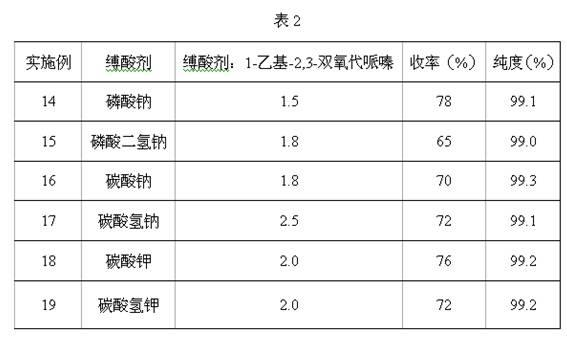
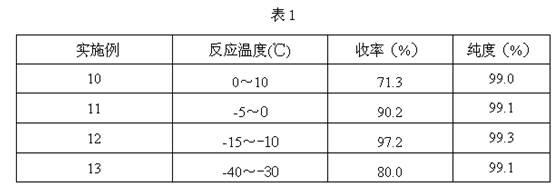
Image
Examples
Embodiment 1
[0018] Add 14.2 g of 1-ethyl-2,3-dioxopiperazine, 100 mL of acetone, and 28.8 mL (0.2 mol) of triethylamine in sequence in the three-necked flask, cool down to -10 °C, and slowly add ethyl chloroformate 11 mL, control the reaction system temperature < -5 ℃. After the dropwise addition, keep warm for 5h. Suction filtration to remove triethylamine hydrochloride, the filter cake was washed once with 15 mL of acetone, the filtrate was collected and decolorized with an appropriate amount of activated carbon, and the solvent was removed under reduced pressure to obtain 4-ethyl-2, 3-dioxopiperazine-1-carboxylic acid Crude ethyl ester. Ethyl acetate was recrystallized to obtain a white powder with a product yield of 95.2% and a purity of 99.3% as determined by HPLC. Example 2
Embodiment 2
[0019] Add 14.2 g of 1-ethyl-2,3-dioxopiperazine, 100 mL of acetone, and 28.8 mL (0.2 mol) of triethylamine in sequence in the three-necked flask, cool down to -10 °C, and slowly add methyl chloroformate 9.3 mL, control the reaction system temperature < -5 ℃. After the dropwise addition, keep warm for 5h. Suction filtration to remove triethylamine hydrochloride, the filter cake was washed once with 15 mL of acetone, the filtrate was collected and decolorized with an appropriate amount of activated carbon, and the solvent was removed under reduced pressure to obtain 4-ethyl-2, 3-dioxopiperazine-1-carboxylic acid Crude methyl ester. Ethyl acetate was recrystallized to obtain a white powder with a product yield of 96% and a purity of 99.1% as determined by HPLC.
Embodiment 3
[0021] Add 14.2 g of 1-ethyl-2,3-dioxopiperazine, 100 mL of dichloromethane, and 34.8 mL (0.25 mol) of triethylamine in sequence in the three-neck flask, cool down to -20 °C, and slowly add ethyl chloroformate dropwise 11 mL, control the temperature of the reaction system < -15 ℃. After the dropwise addition, keep warm for 5h. Suction filtration to remove triethylamine hydrochloride, the filter cake was washed once with 15 mL of acetone, the filtrate was collected and decolorized with an appropriate amount of activated carbon, and the solvent was removed under reduced pressure to obtain 4-ethyl-2, 3-dioxopiperazine-1-carboxylic acid Crude ethyl ester. 1:1 (volume ratio) acetone / petroleum ether was recrystallized to obtain a white powder with a product yield of 95% and a purity of 99.4% as determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com