Industrial production method for clindamycin or salts thereof
A technology of clindamycin and lincomycin, applied in the direction of sugar derivatives, organic chemistry, etc., can solve the problems of low yield, cumbersome operation, HPLC purity rarely reaches 90%, etc. The effect of easy handling and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
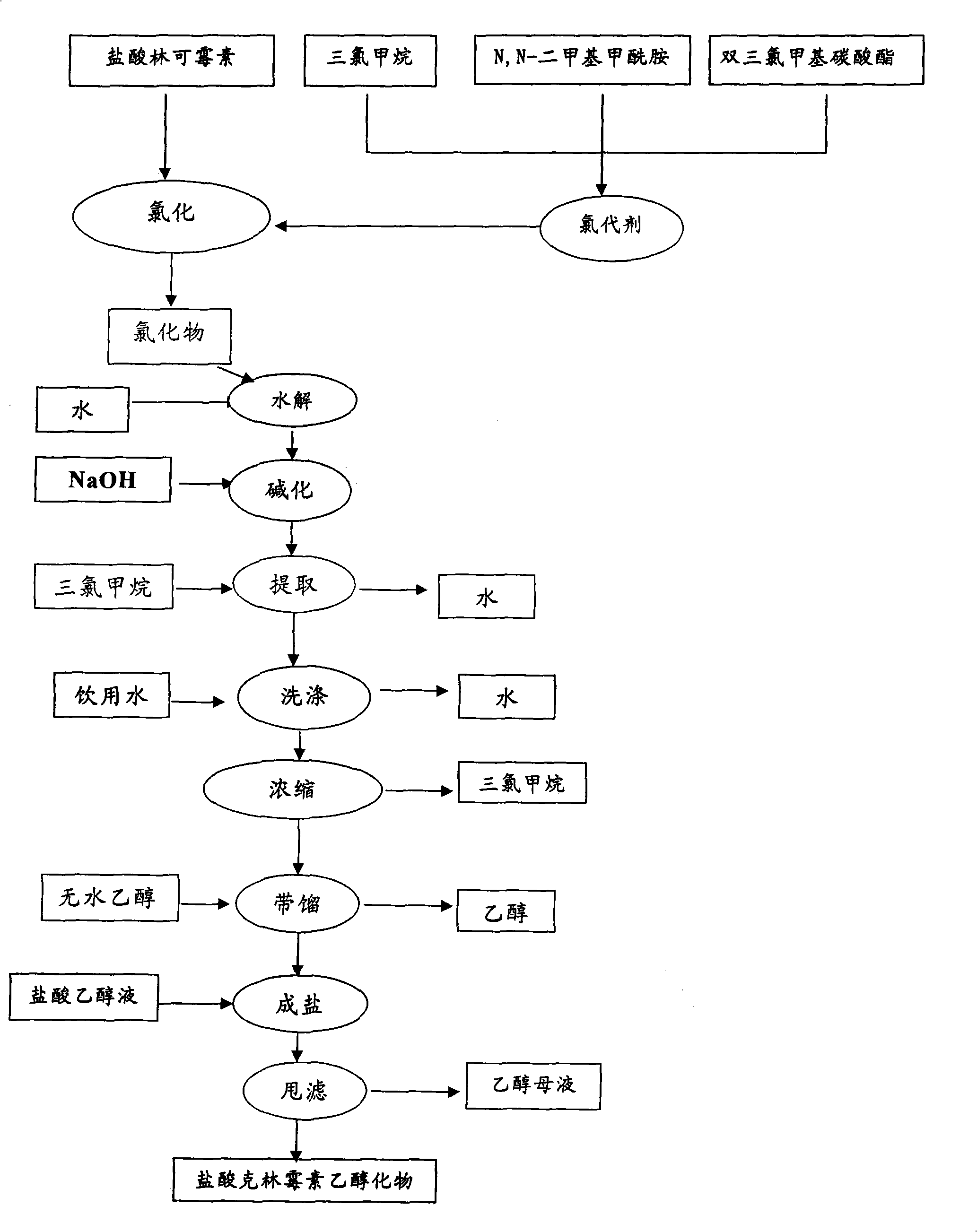
[0048] 1. Chloride Preparation
[0049] 1.1 Chlorinating agent [(CH 3 ) 2 N=CHCl + ] Cl - preparation of
[0050] At 25±5°C, pump 270kg of chloroform into the reactor, add 115kg of N,N-dimethylformamide, and pump it into the high-level tank. Then add 375 kg of chloroform and 126.5 kg of bis-trichloromethyl carbonate into the main reaction pot, blow nitrogen, and when the ice brine drops to 0°C, start to add the N,N-dimethylformamide prepared above dropwise. Chloroform solution of amide, maintain the internal temperature at 10±2°C during the dropwise addition. After dropping, stir for 1 hour, heat up to an internal temperature of 15-25°C and react for 0-3 hours, cool down in ice-water solution to obtain the chlorinated agent [(CH 3 ) 2 N=CHCl + ] Cl - solution.
[0051] 1.2 Preparation of Chloride
[0052]When the internal temperature dropped below -2°C, 100 kg of lincomycin hydrochloride was added under the protection of nitrogen, and the interlayer was maintained t...
Embodiment 2
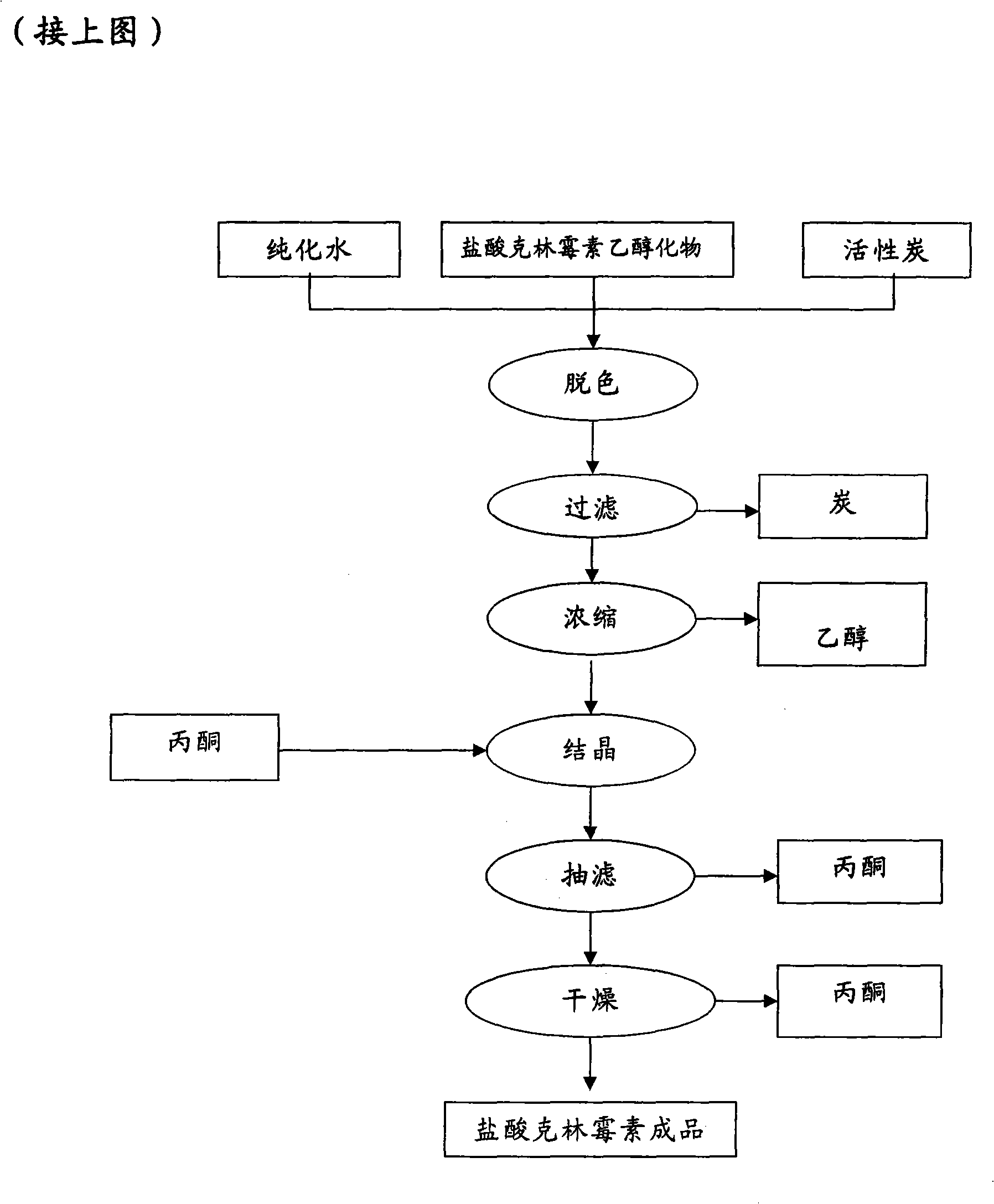
[0072] According to the method of embodiment 1, just replace 126.5kg bis-trichloromethyl carbonate with 150kg trichloromethyl chloroformate, obtain clindamycin hydrochloride, HPLC purity 97.8%, total molar yield is calculated with respect to lincomycin hydrochloride 78% to 86%.
Embodiment 3
[0074] According to the method of embodiment 1, just replace 100kg lincomycin hydrochloride with 95kg lincomycin free base, obtain clindamycin hydrochloride, HPLC purity>98%, total molar yield is calculated as to lincomycin free base 80% to 87%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com