Phototoxicity detection method based on recombinant skin model
A technology of skin model and detection method, which is applied in the field of skin phototoxicity detection and research, can solve the problems of limited test conditions and phototoxicity detection, and achieve the effect of expanding the detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
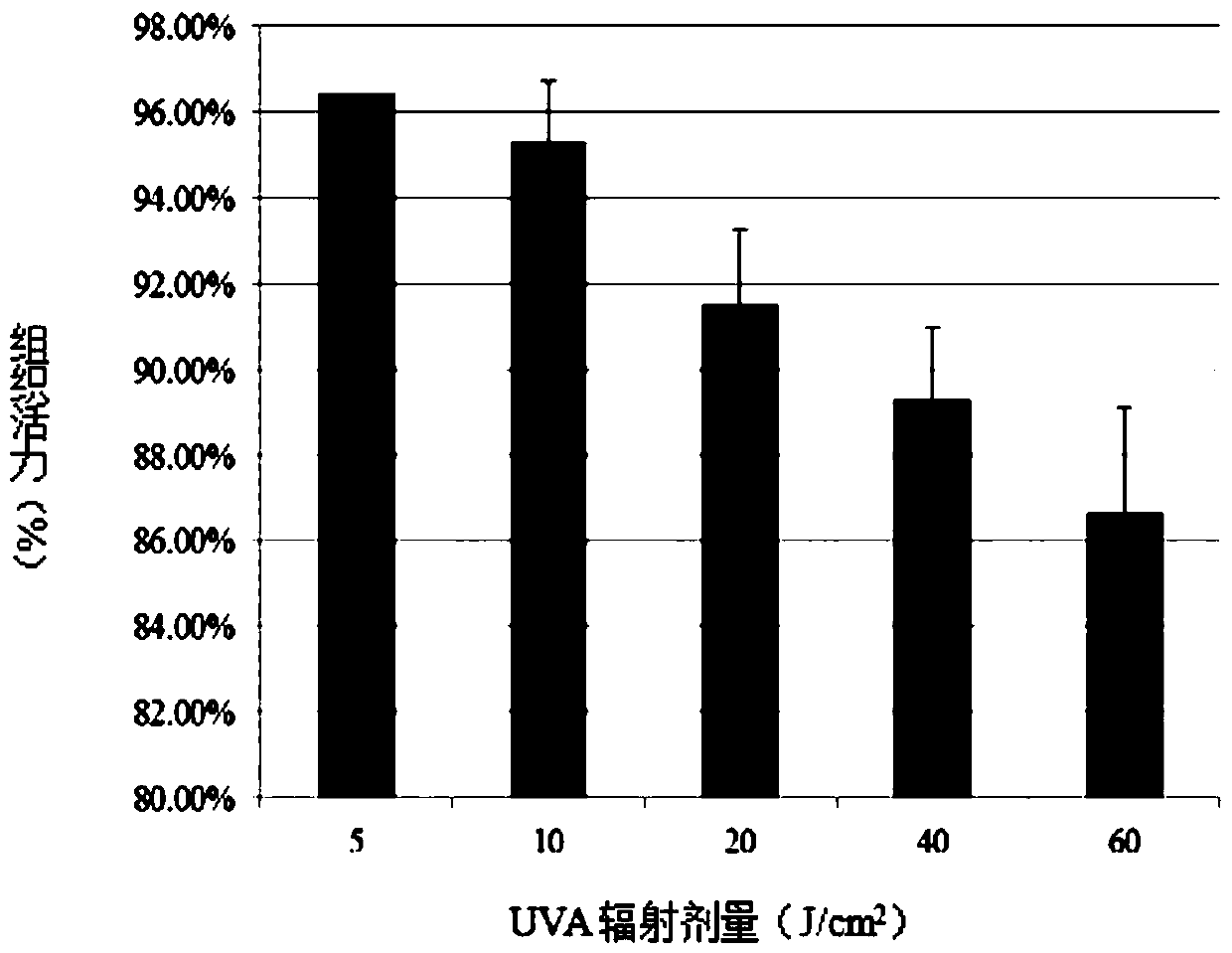
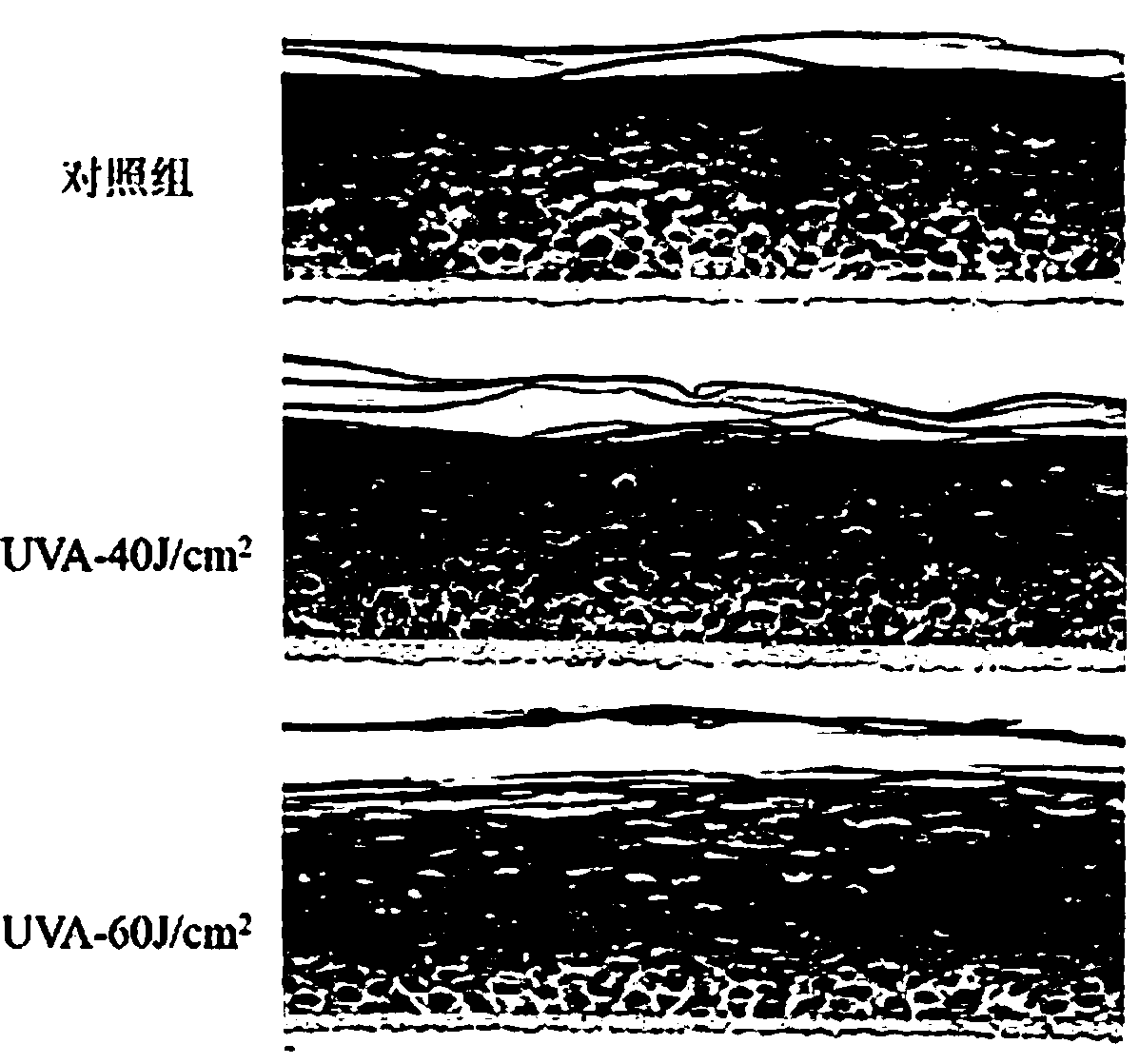
[0045] The present embodiment provides a phototoxicity evaluation method based on the recombinant skin model Epikutis, comprising the following steps:
[0046] S1. Receiving and resuscitating the reconstructed skin model Epikutis;
[0047] S2. Select a light source;
[0048] S3. Determine the maximum tolerated dose of the recombinant skin model Epikutis;
[0049] S4. Detect the phototoxicity of the test substance.
[0050] Said step S1 comprises:
[0051] Culture the recombinant skin model Epikutis in a 6-well plate, 1 recombinant skin model Epikutis in each well, add 900 μL resuscitation solution per well, 36-38°C, 4-6% CO 2 Incubate in an incubator with 95% relative humidity for 55-65min, then replace the resuscitation solution, and incubate overnight for 18-22h. During the resuscitation process, discard the reconstructed skin model Epikutis that is damaged and has a large amount of water on the surface. The appearance of the reconstructed skin model Epikutis was evaluat...
Embodiment 2
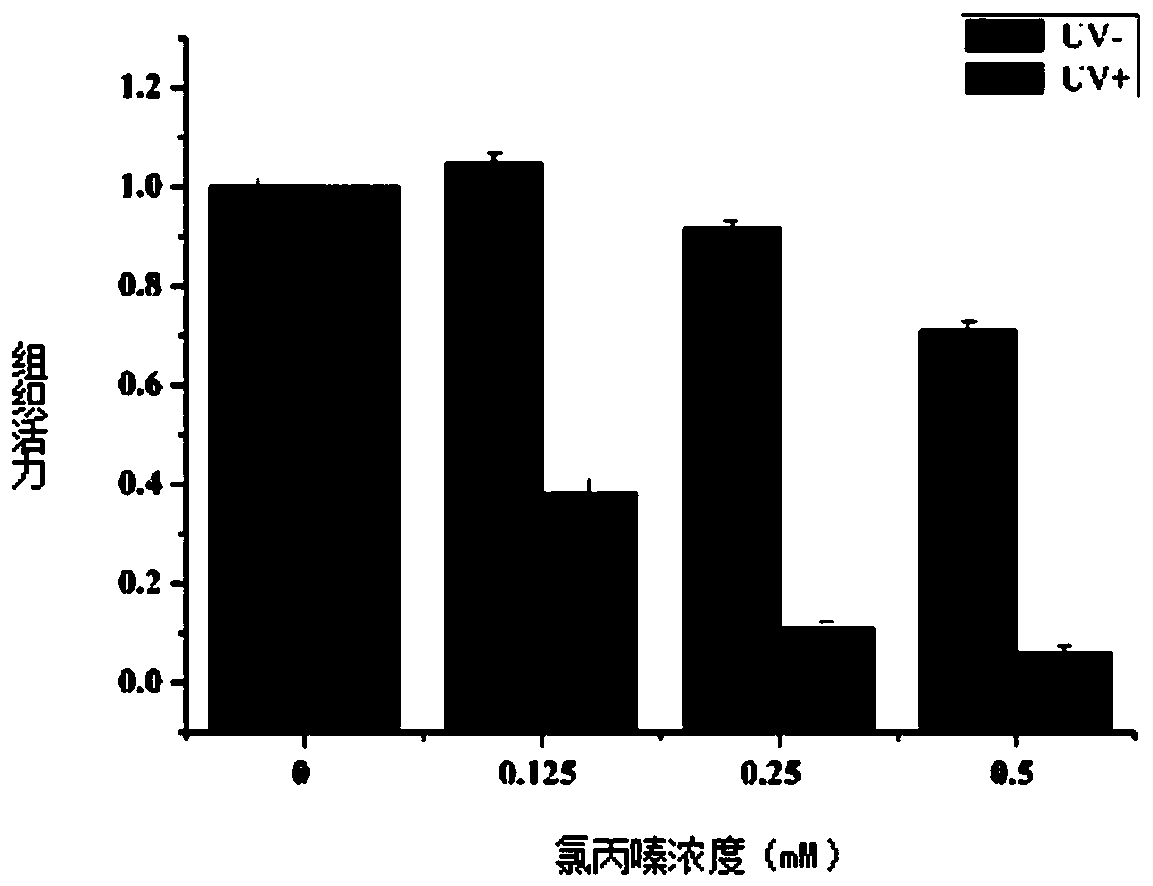
[0067] The present embodiment provides a phototoxicity evaluation method based on the recombinant skin model Epikutis of a chemical chlorpromazine, comprising the following steps:
[0068] S1. Receiving and resuscitating the reconstructed skin model Epikutis;
[0069] S2. Select a light source;
[0070] S3. Determine the maximum tolerated dose of the recombinant skin model Epikutis;
[0071] S4. Detect the phototoxicity of the test substance.
[0072] Said step S1 comprises:
[0073] Culture the recombinant skin model Epikutis in a 6-well plate, 1 recombinant skin model Epikutis in each well, add 900 μL resuscitation solution per well, 36-38°C, 4-6% CO 2 Incubate in an incubator with 95% relative humidity for 55-65min, then replace the resuscitation solution, and incubate overnight for 18-22h. During the resuscitation process, discard the reconstructed skin model Epikutis that is damaged and has a large amount of water on the surface. The appearance of the reconstructed sk...
Embodiment 3
[0086] This example provides a phototoxicity evaluation method based on the recombinant skin model Epikutis for a finished cosmetic product F) [4], including the following steps:
[0087] S1. Receiving and resuscitating the reconstructed skin model Epikutis;
[0088] S2. Select a light source;
[0089] S3. Determine the maximum tolerated dose of the recombinant skin model Epikutis;
[0090] S4. Detect the phototoxicity of the test substance.
[0091] Said step S1 comprises:
[0092] Culture the recombinant skin model Epikutis in a 6-well plate, 1 recombinant skin model Epikutis in each well, add 900 μL resuscitation solution per well, 36-38°C, 4-6% CO 2 Incubate in an incubator with 95% relative humidity for 55-65min, then replace the resuscitation solution, and incubate overnight for 18-22h. During the resuscitation process, discard the reconstructed skin model Epikutis that is damaged and has a large amount of water on the surface. The appearance of the reconstructed ski...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com