Cancer treatments with radiation and immunocytokines
A technology of immune cells and cytokines, which is applied in the field of treating tumors and cancer cells, and can solve problems such as no improvement and dyspnea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
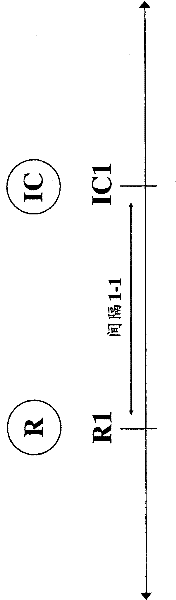
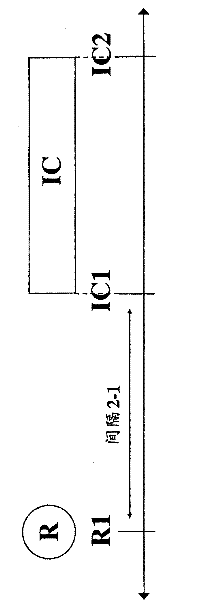
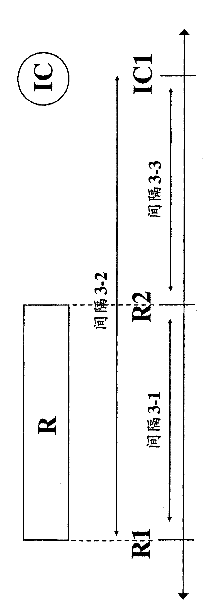
[0080]In accordance with the methods of the present invention, third parties, such as hospitals, clinics, government entities, reimbursers, insurance companies (e.g., health insurance companies), HMOs, third-party payers, or other entities that pay for or reimburse medical expenses may approve treatment, approval as Payment for treatment or approval for reimbursement for treatment. For example, the invention relates to health care methods that include authorizing administration of an immunocytokine to a mammal having previously irradiated tumor or cancer cells, or authorizing payment or reimbursement for administration of an immunocytokine. For example, the health care method may include an approved dose of at least 1 Gy / day (e.g., at least 2, at least 3, 1-4, 1-10, 1-20, 2-4, 2-10, 2-20, 3-4, 3-10 or 3-20 Gy / day), administration of immunocytokine to mammals with previously irradiated tumors, or approval of payment or reimbursement for administration of immunocytokine. The he...
Embodiment 2
[0097] Student's two-tailed t-test or Mann-Whitney rank sum test was performed on individual tumor volumes or animal body weights, as appropriate, to determine significant differences between treatment groups. Example 2: Effect of Immunocytokine Therapy After a Single Dose of Radiation
[0098] A single dose of post-radiation intravenous immunocytokine (5 mg / kg). Unless otherwise specified, this and the following examples use the exemplary immunocytokine dI-NHS76γ2(h)(FN>AQ)-ala-IL2(D20T), also named Selectikine or EMD521873, and described in, e.g. U.S. Patent No. 7,186,804.
[0099] In three separate experiments using the CT26 / KSA model (experiments CT26-1-3), administration of EMD521873 at days 2, 3 and 4 following tumor irradiation with 3 or 4 Gy resulted in a strong synergistic effect, with most animals achieving complete subside. ELISPOT analysis using endogenous CT26 antigen showed a >4-fold increase in T cell responses compared to either therapy alone. A 4-fold in...
Embodiment 3
[0101]The antitumor effect of an exemplary immunocytokine EMD521873 (5 mg / kg) administered intravenously on days 7, 8, and 9 following five daily doses of 3.6 Gy on days 0 to 4 was evaluated in a Lewis lung cancer model (Experimental LLC-1, LLC-2). No complete responses were observed in the control or monotherapy groups, whereas 3 / 6 animals achieved a complete response with the combination. Gene expression profiling using a panel of immune markers showed that the combination increased T-cell markers, T-cell activation, lymphocyte trafficking, and Th1 responses compared to radiation or EMD521873 alone. Upregulated genes include CD45, CISH, CD122, MGP, FASL, CD80, PTPRB, CD6, CCR7, TXK, CTLA4, PDCD1, IL10R, CCL6, CD8A, EOMES, CD28, TγROBP, ICAM1, CD206, VCAM1, CD3G, ITGAL, ITGB2, LAT, GZMK, STAT4, IL1A, CD115, MDM2, CD26, GIMAP3, CXCR4, LCK, HS6ST2. Downregulated genes included IL23A, SELE, SC4MOL, LDLR, SQLE, RAE1, CXCL1, CCL2. Example 4: Effect of Adding Chemotherapy to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com