Kit for detecting lncARSR in serum and application of kit in detection of kidney cancer sunitinib drug resistance
A detection kit and detection reagent technology, applied in the field of medical biological detection, can solve the problem that the value of serological markers has not been reported in literature, achieve a good prospect of translational medicine, and avoid the effect of ineffective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Screening and identification of lncARSR
[0042] We previously constructed sunitinib-resistant kidney cancer cell lines 7SuR and ACSuR (preservation numbers: CCTCCC2014217, CCTCCC2014238), and have applied for the Chinese invention patent CN201510304372.8, the invention name is "kidney cancer sunitinib resistance Cell lines and methods for their construction".
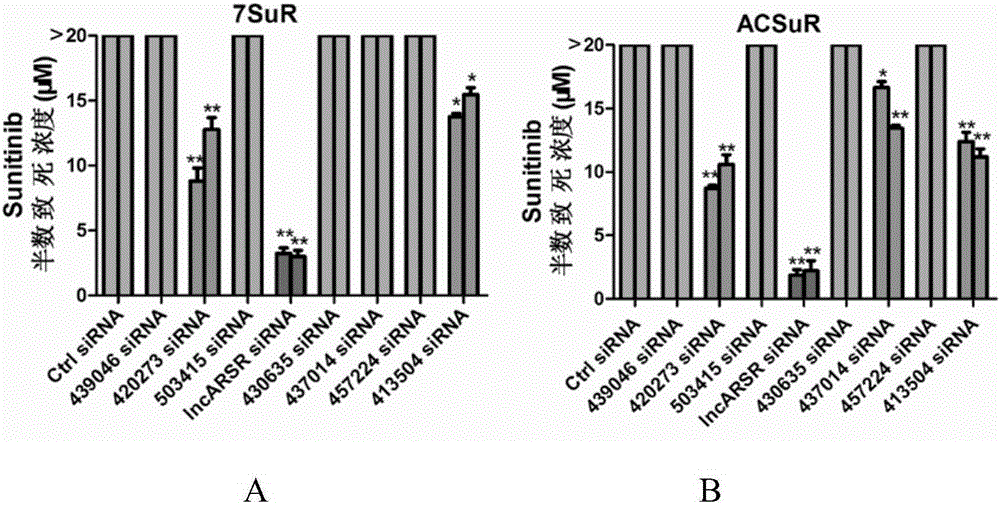
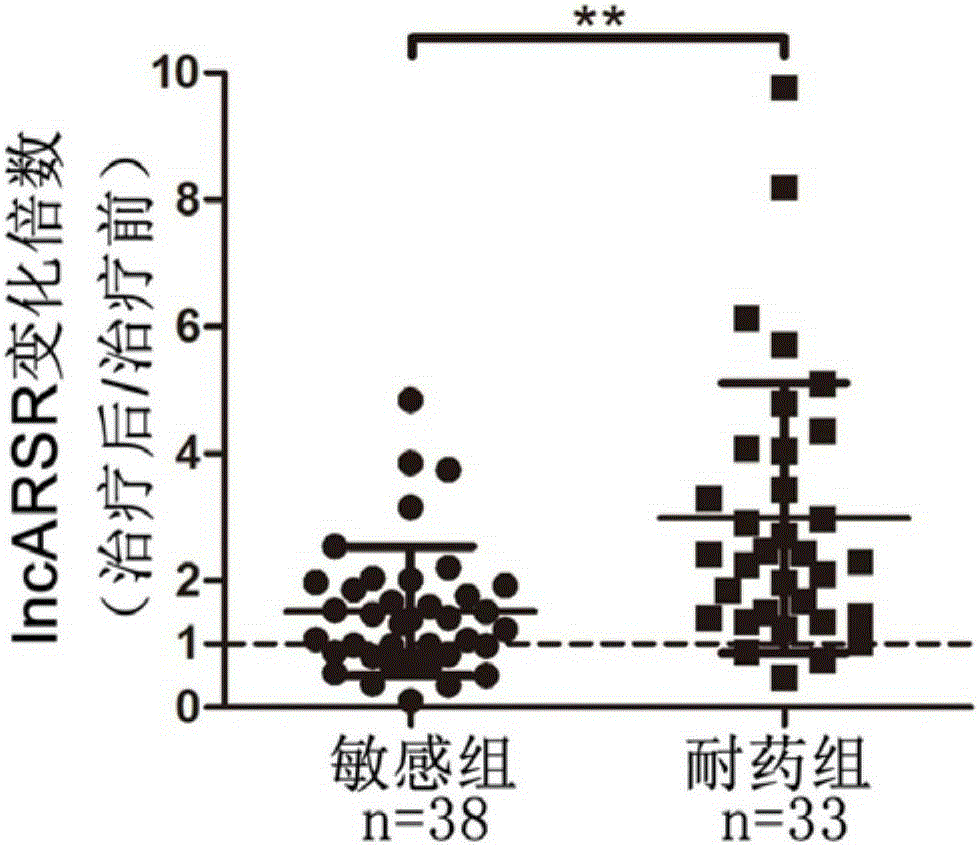
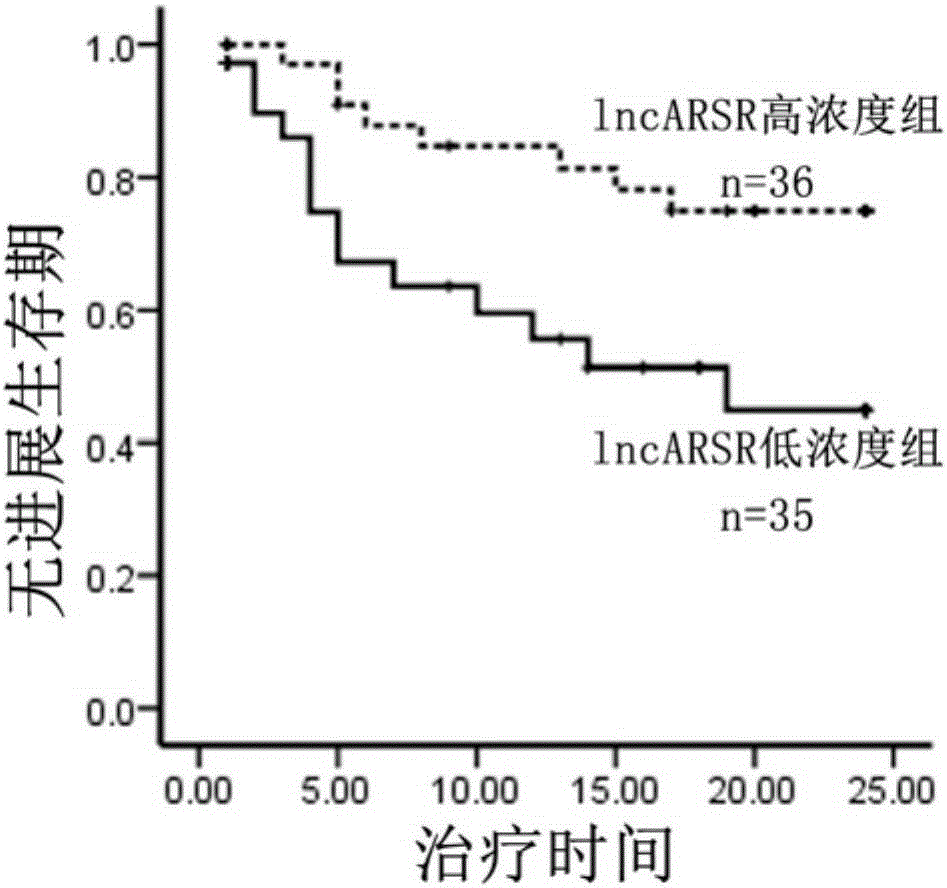
[0043] In order to study lncRNAs related to sunitinib resistance, we used lncRNA microarray analysis on renal cancer parental cells and drug-resistant cells, and screened 8 most significantly elevated lncRNAs. Further, we used RNAi technology to verify the function of 8 lncRNAs, and found that interfering with lncARSR restored the sensitivity of drug-resistant cells to sunitinib ( figure 1 ). The above results suggest the role of lncARSR in RCC sunitinib resistance, and also suggest that it may have application value as a diagnostic marker for sunitinib resistance.
Embodiment 2
[0044] Example 2: Collection and processing of serum samples and extraction of small RNAs
[0045] (1) Serum sample collection: Collect about 6ml of peripheral blood from kidney cancer patients and put it into an anticoagulant tube containing EDTA, and let it stand for about 1 hour.
[0046] (2) Serum sample processing: the collected peripheral blood samples were centrifuged at 4000 rpm for 10 min at 4°C, the supernatant was taken and packed into EP tubes, and stored at -80°C.
[0047] (3) Extraction of RNA in serum: 350 μl of each serum sample was taken, 1 μl of exogenous standard RNA (λpolyA+RNA-A) (purchased from TAKARA Company) was added, and then mirVana purchased from Ambion Company was used to TM PARIS TM Kit to extract small RNA.
[0048](4) Reverse transcription into cDNA: take the same amount of RNA from each sample, and perform reverse transcription with random primer N6. According to 5×M-MLVRTBuffer 5 μl, M-MLVRversetranscriptase 1 μl, rRNasin 0.5 μl (Promega Co...
Embodiment 3
[0049] Example 3: Primer Design and Verification
[0050] (1) Primer design: use the Ensemble database to check the full-length sequence of lncARSR (ENST00000424980), and use PrimerPremier5 software to design a pair of upstream and downstream primers for Real-timePCR for the sequence of lncARSR, as follows:
[0051] Upstream primer: TTTGAAATGCTCTTTGAGGGAT (SEQ ID NO: 1);
[0052] Downstream primer: TGCAGGTTGTCTGAAGTTGGA (SEQ ID NO: 2);
[0053] The primers were synthesized by Shanghai Sangong Company.
[0054] (2) Primer verification: SYBRPremixExTaq from TaKaRa Company TM Reagents and the ViiA7Dx real-time quantitative PCR instrument of Applied Biosystems in the United States were used to carry out the PCR reaction according to the following system:
[0055]
[0056] PCR conditions:
[0057]
[0058] According to the above Real-timePCR conditions, the lncARSR primers were detected by using the reverse transcription products of 8 serum samples prepared earlier. Two ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com