Method for synthesizing sunitinib alkali
A technology of sunitinib base and synthesis method, which is applied in the directions of drug combination, organic chemistry, antitumor drugs, etc., can solve the problems of incomplete hydrolysis, and achieve the effects of reduced cost, high yield and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
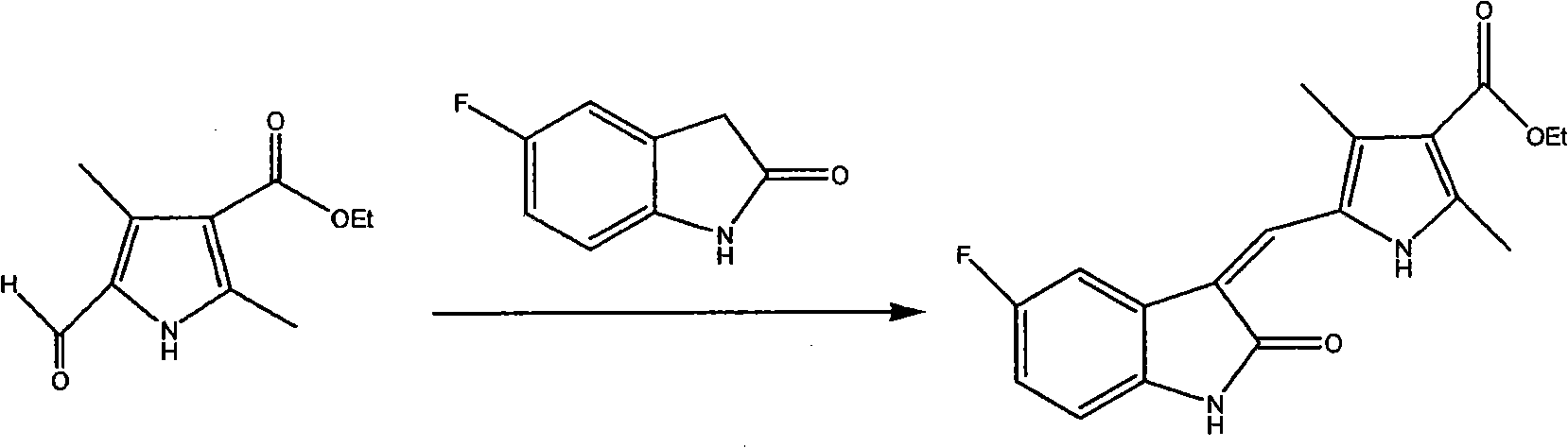
[0029] 0.70 g (3.59 mmol) of ethyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate, 0.45 g (3 mmol) of 5-fluoroindol-2-one, 9mL of ethanol and several drops of piperidine were reacted at 80°C for 3 hours under nitrogen protection, and a large amount of solids appeared. After cooling, filter with suction, wash the filter cake with ethanol, and vacuum-dry to obtain 0.88 g of solid, with a yield of 89.3%.
[0030] Appearance: yellow solid
[0031] Melting point: 284-286°C
[0032] 1H-NMR (DMSO2-d6) δ: 1.29(t, 3H), 2.422(s, 3H), 2.442(s, 3H), 4.203(q, 2H), 6.847(q, 1H), 6.947(t, 1H ), 7.762 (s, 1H, vinylH), 7.801 (d, 1H), 10.948 (s, 1H, indoleNH), 13.921 (s, 1H, pyrroleNH).
Embodiment 2
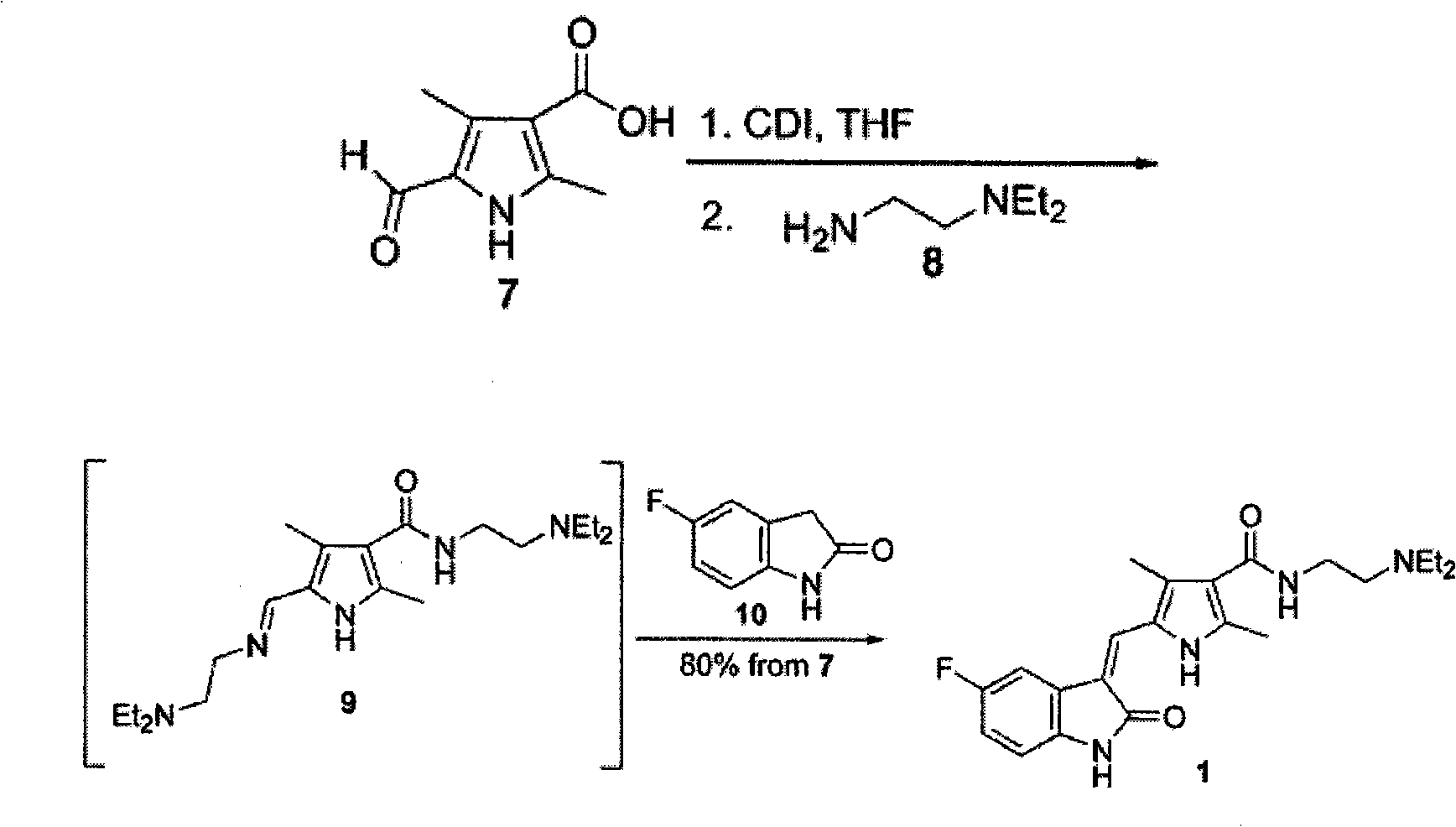
[0034] 5-((5-fluoro-2-oxoindol-3-enyl)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl ester 0.8g (2.5mmol), 2 -(Diethylamino)ethylenediamine 0.5ml, catalyst 0.05g, xylene 20ml, react at 100°C for 12h, cool down, add chloroform to dilute, filter, evaporate xylene under reduced pressure to obtain solid 0.81g, yield 73.2%.
[0035] Appearance; orange-yellow solid
[0036] Melting point: 214-216°C
[0037] 1H-NMR (DMSO2-d6) δ: 0.977(t, 6H), 2.420(s, 3H), 2.440(s, 3H), 2.518~2.556(m, 6H), 3.275(m, 2H), 6.837(br s, 1H), 6.923 (dd, 1H), 7.423 (m, 1H), 7.712 (s, 1H), 7.764 (dd, 1H), 10.873 (s, indole NH), 13.676 (s, pyrrole NH).
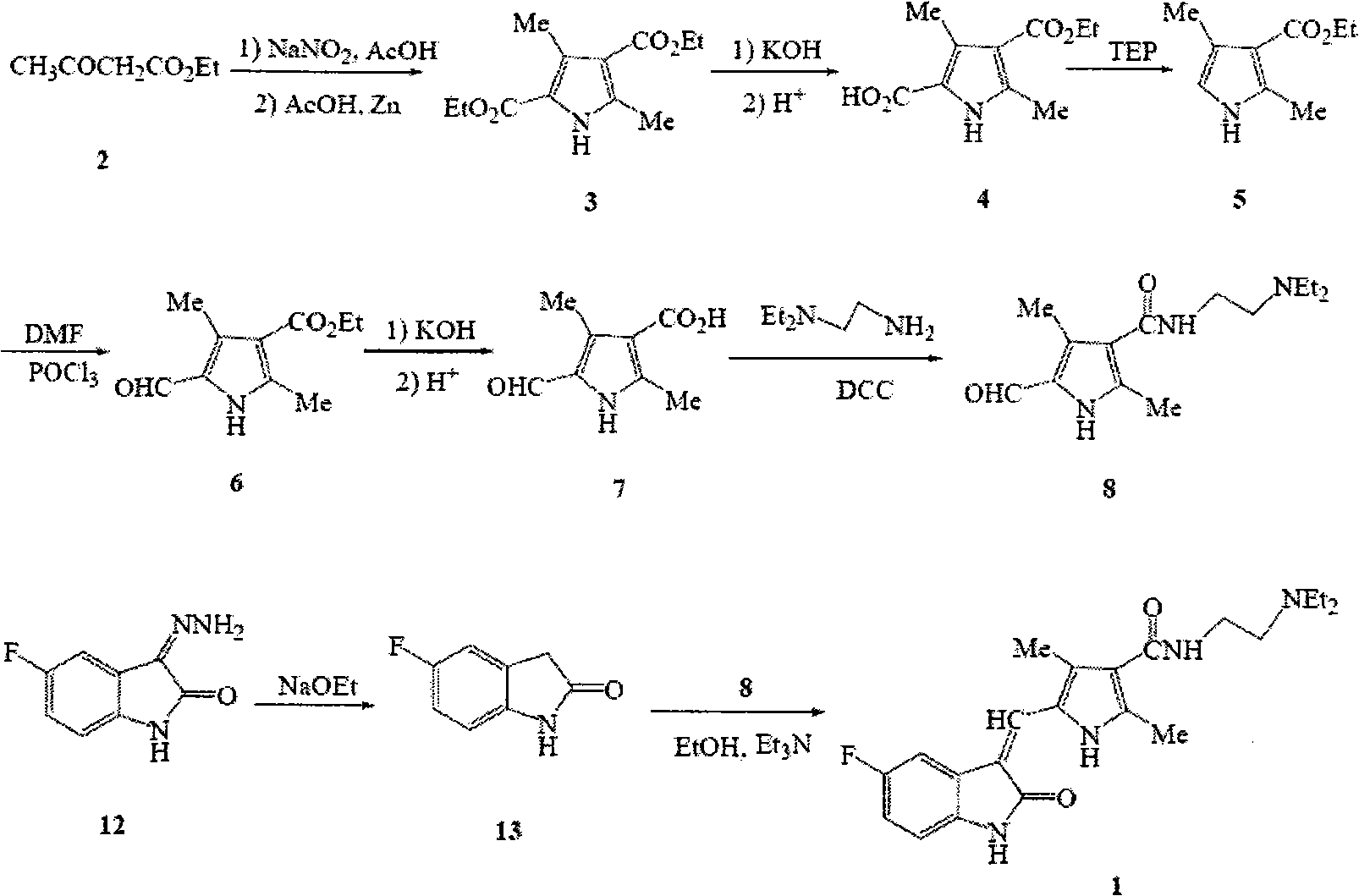
[0038] Starting from ethyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate, the traditional three-step reaction is combined into two, and the yield of each step is high, which greatly improves the reaction yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com