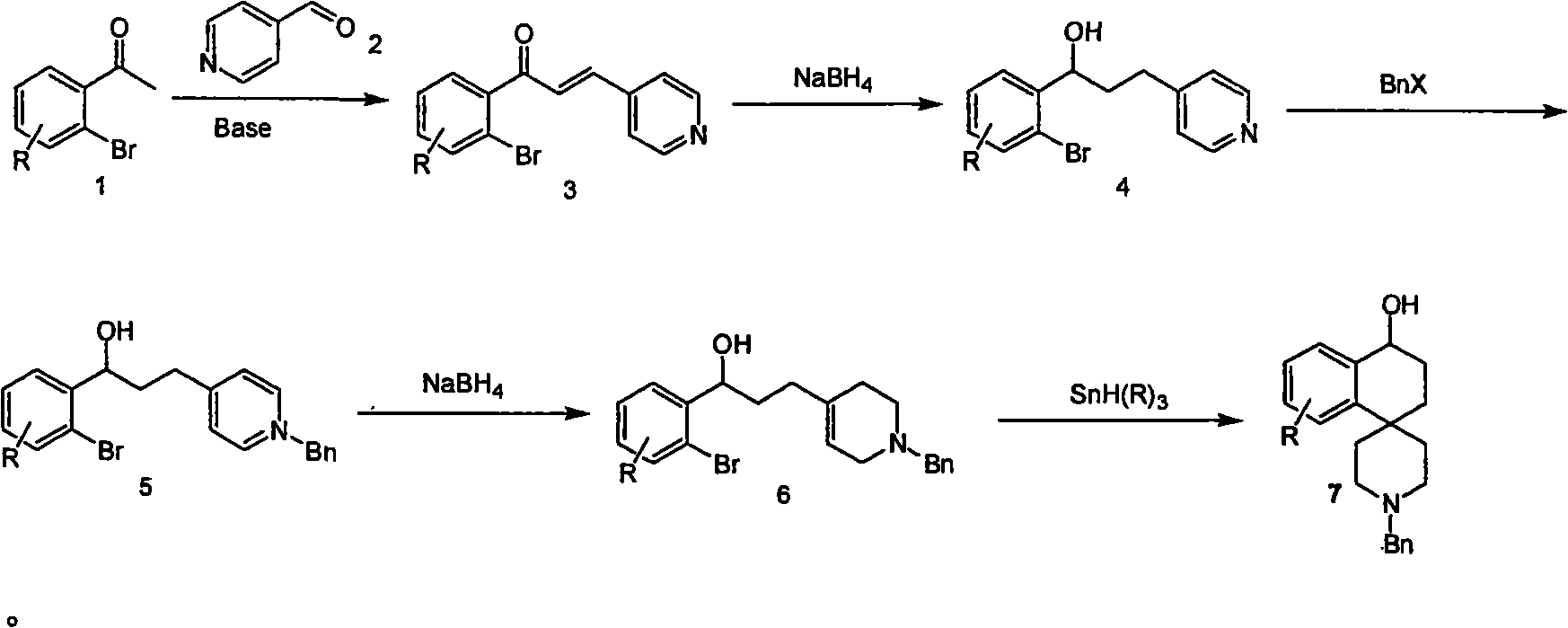
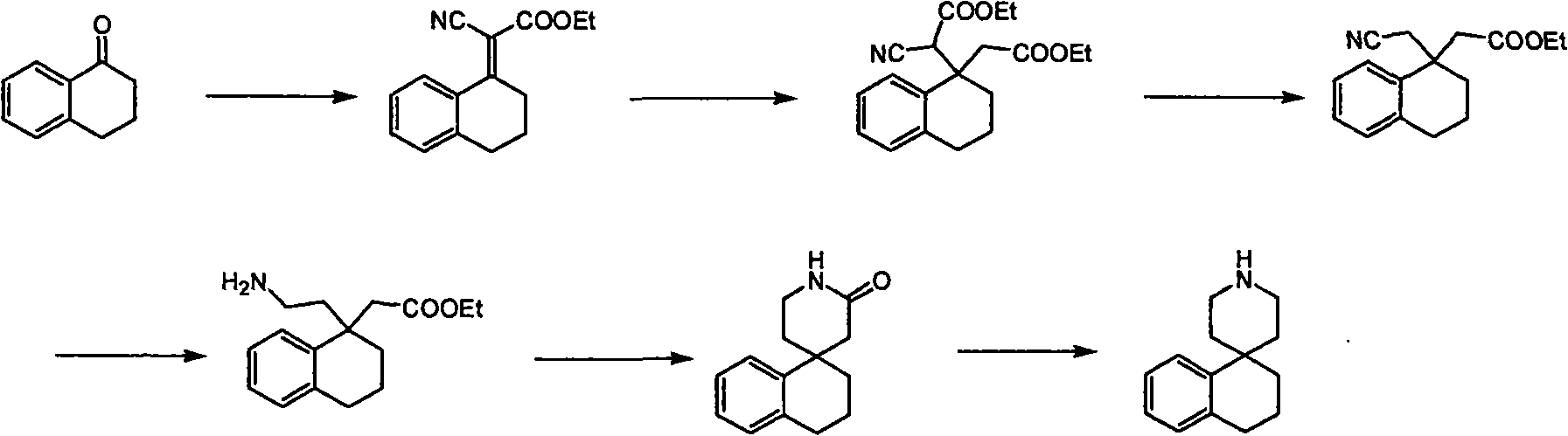
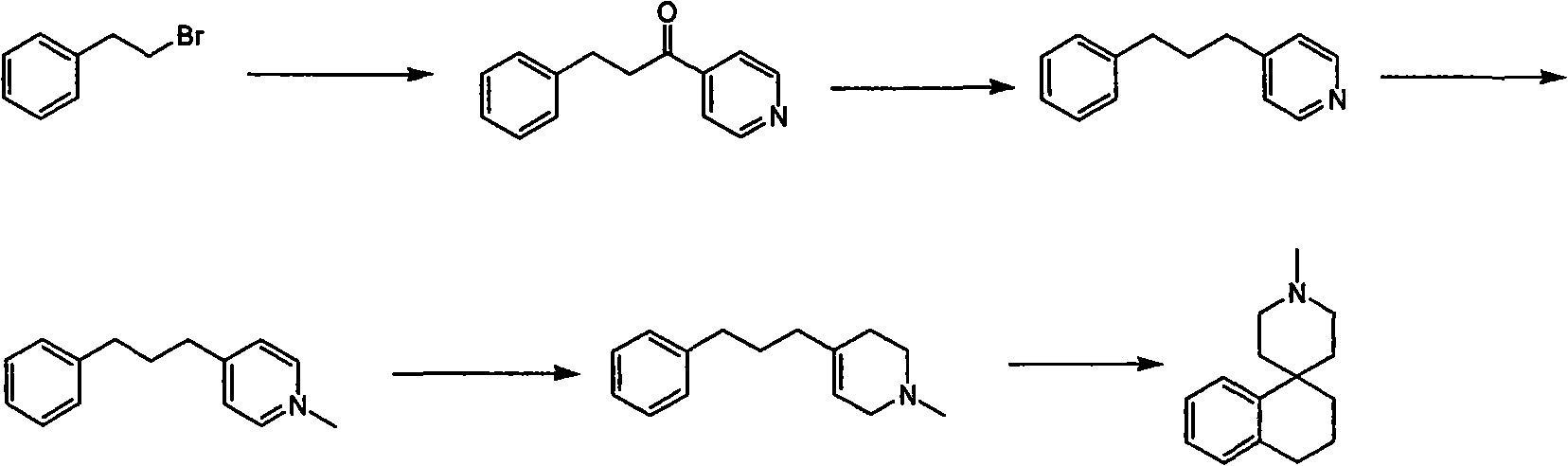
Synthesis of whorl(1-tetrahydronaphthalene-4,4'-piperidine) derivative
A synthetic method and technology of tetrahydronaphthalene, applied in the field of synthesis of spiro derivatives, achieve the effects of short synthetic route, high yield and convenient reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. The preparation of compound (3):
[0018]
[0019] Add 20 ml of 5% aqueous sodium hydroxide solution and 10 ml of methanol into the reaction flask, and cool in an ice-water bath. 10 grams of o-bromoacetophenone was added to the reaction flask. After the addition was complete, 10 grams of 4-pyridinecarbaldehyde was added dropwise to the reaction over 90 minutes. After the addition was complete, the reaction was carried out at this temperature for 1.5 hours. After the reaction, water and tert-butyl methyl ether were added to separate the layers. The organic layer was washed with water and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated to dryness to obtain the product compound 3 (8.6 g, Yield 60%).
[0020] 1H (300MHz, CDCl3) δ8.71-8.6(m, 2H), 7.6(d, J=4Hz, 1H), 7.48-7.36(m, 6H), 7.31(s, 1H), 7.28(s, 1H) .
[0021] 2. Preparation of compound (4):
[0022]
[0023] Ethanol (100 ml) and compound 3 (8.6 g) were added to th...
Embodiment 2
[0037] According to the reaction formula in the technical scheme, the alkali in the first step reaction is potassium carbonate, the solvent used is water, and the reaction temperature is -20-80°C; the second step reaction solvent is dichloromethane, and the reaction temperature is from room temperature to the reflux of the solvent temperature. In the third step, the reaction solvent is tetrahydrofuran; the base is sodium carbonate; the reaction temperature is from room temperature to the reflux temperature of the solvent; the benzyl halide of the alkylating reagent is benzyl chloride. The fourth step reaction solvent is dichloromethane; the reaction temperature is from room temperature to the reflux temperature of the solvent. The fifth step reaction solvent is benzene. All the other are identical with embodiment 1.
Embodiment 3
[0039] According to the reaction formula in the technical scheme, the alkali in the first step reaction is sodium carbonate, the solvent used is methanol and water, and the reaction temperature is -20-80°C; the second step reaction solvent is tetrahydrofuran, and the reaction temperature is from room temperature to the reflux of the solvent temperature. The third step reaction solvent is toluene; the base is sodium hydroxide; the reaction temperature is from room temperature to the reflux temperature of the solvent; the alkylating reagent benzyl halide is benzyl chloride. The fourth step reaction solvent is benzene; the reaction temperature is from room temperature to the reflux temperature of the solvent. The fifth step reaction solvent is toluene. All the other are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com