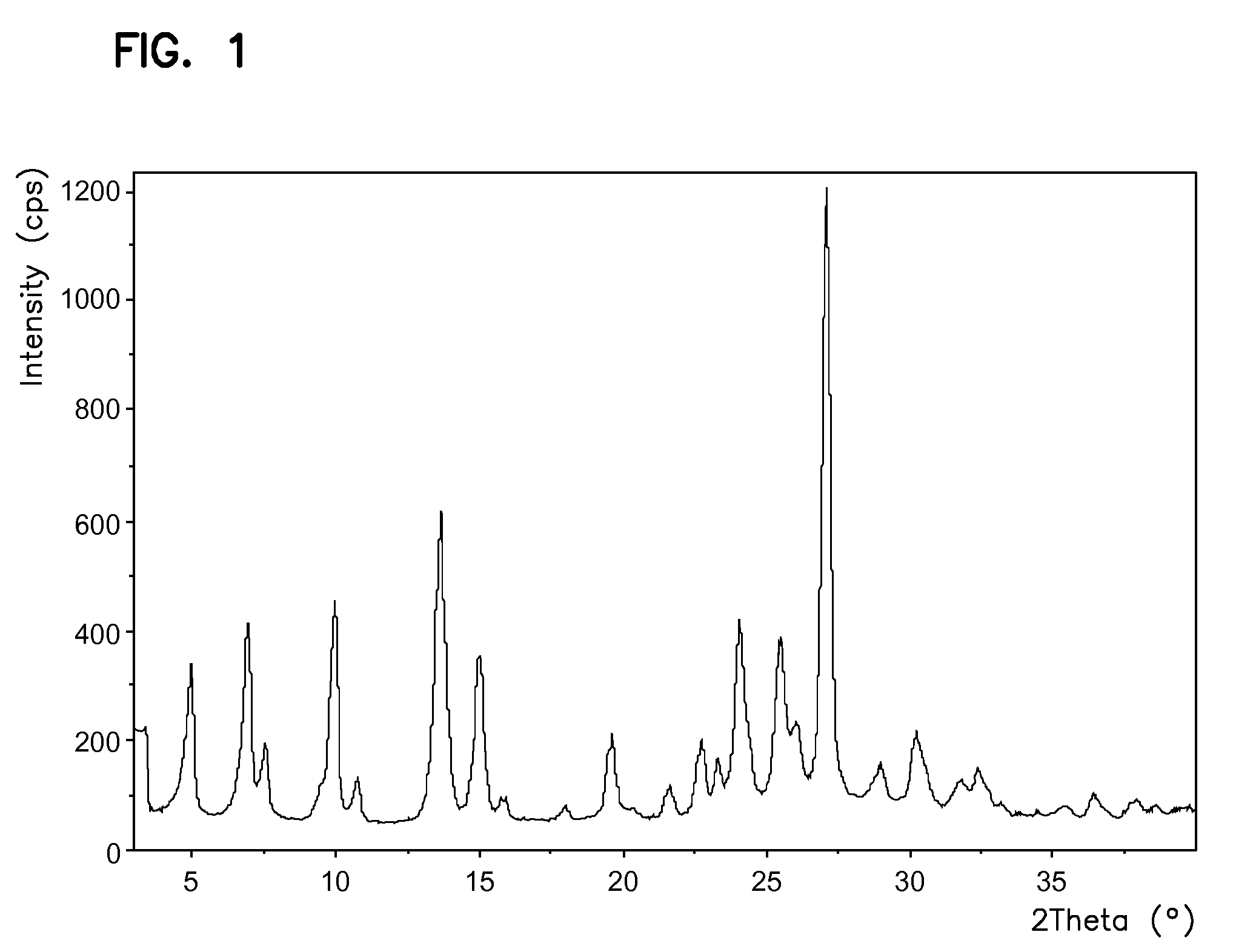
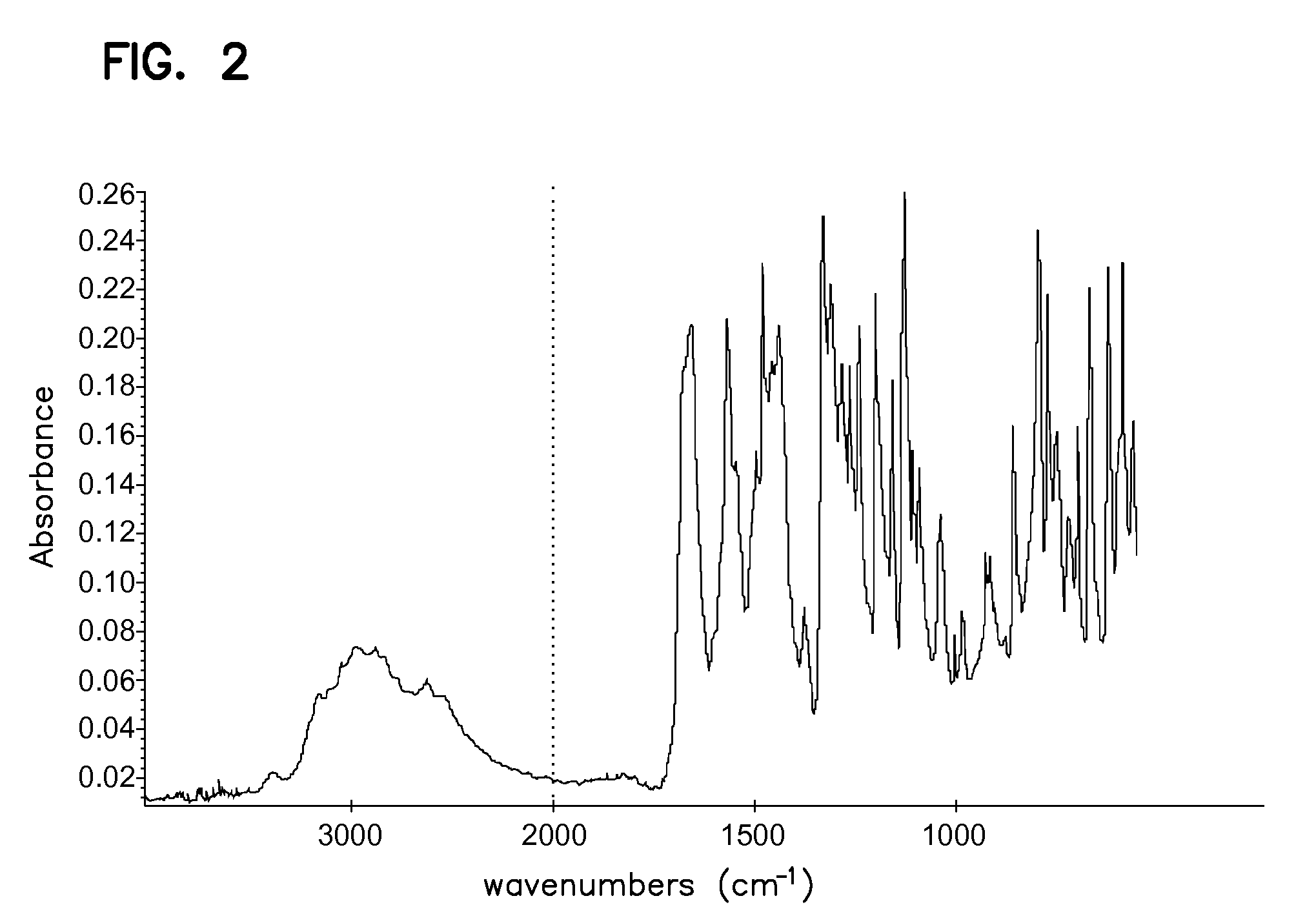
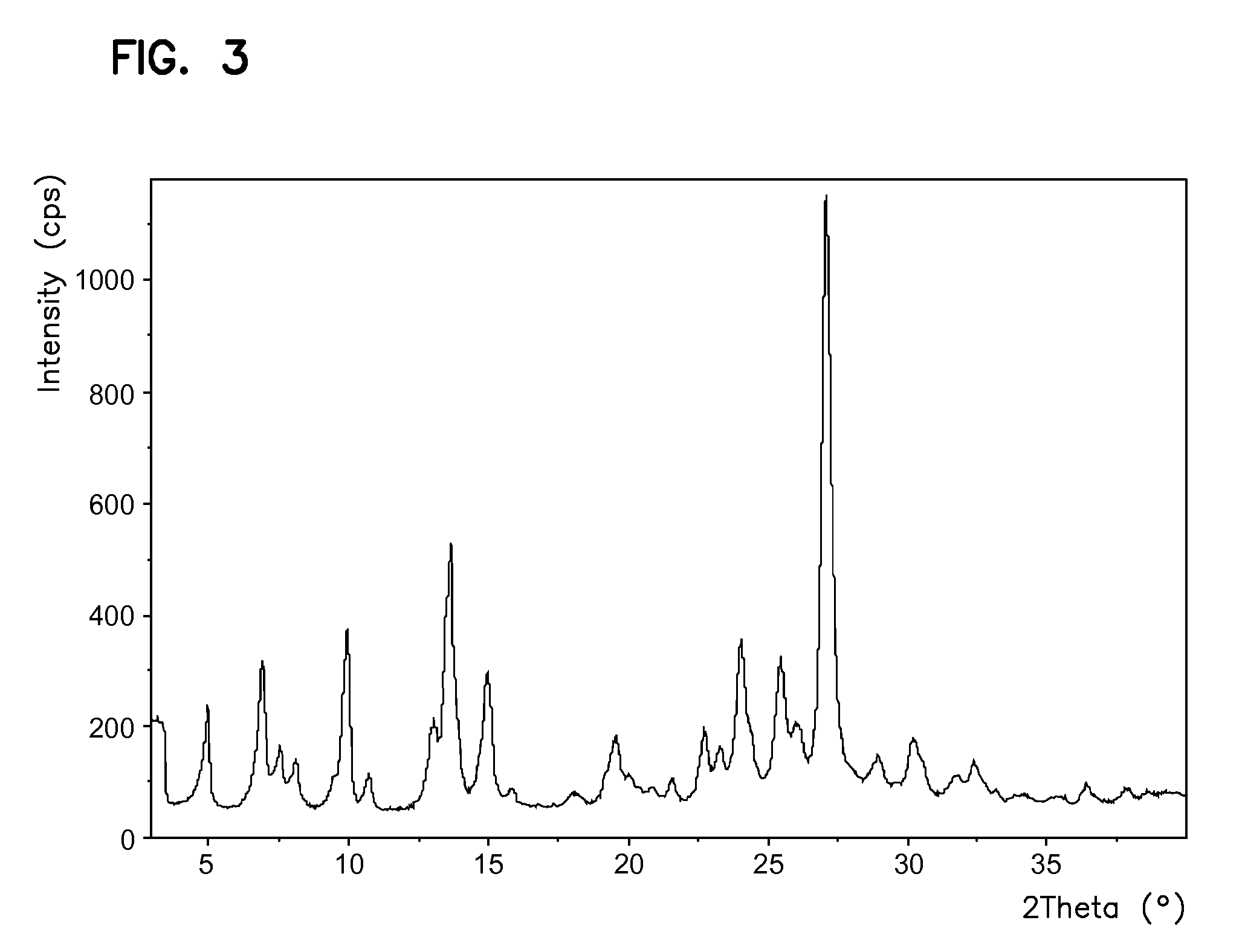
Processes for preparing sunitinib and salts thereof
a technology which is applied in the field of process for the preparation of sunitinib and salt thereof, can solve the problems of toxic, dangerous, expensive reagents,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of sunitinib via 5-(5-fluoro-2-oxo-1,2-dihydro-indol-3Z-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carbonyl chloride
[0106]31.2 g of 5-(5-fluoro-2-oxo-1,2-dihydro-indol-3Z-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid, obtained as described in U.S. Pat. No. 7,125,905, were refluxed under stirring for 4 hours in one liter flask with 310 g of toluene, 15 g of thionyl chloride and 1 g of dimethylformamide.
[0107]The stirred suspension was cooled at room temperature for 2 hours and filtered; the cake was washed with 50 g of toluene and dried at 50° under vacuum overnight.
[0108]Yield was 32.4 g (97.8%) of a compound corresponding by NMR and MS to the expected structure.
[0109]20 g of diethylendiamine were dissolved in one liter flask with 300 g of tetrahydrofuran; about 200 g of solvent were distilled away at 50° under vacuum.
[0110]20 g of 5-(5-fluoro-2-oxo-1,2-dihydro-indol-3Z-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carbonyl chloride, prepared as above, were added und...
example 2
Preparation of Sunitinib via 5-(5-fluoro-2-oxo-1,2-dihydro-indol-3Z-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-(carbonyl-1-imidazole)
[0114]4.6 g of 5-(5-fluoro-2-oxo-1,2-dihydro-indol-3Z-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid, obtained as described in U.S. Pat. No. 7,125,905, were stirred for 4 hours in 0.1 liter flask with 46 g of 1-methyl-2-pyrrolidone and 3 g of 1,1′-carbonyldiimidazole (CDI), after this time 0.7 g of CDI were added and reaction was left stirring overnight.
[0115]46 g of water were added under stirring and after 1 hour the suspension was filtered and the cake washed with water.
[0116]Product was dried at 60° under vacuum obtaining 5.1 g (95% yield); NMR and MS confirmed the expected structure.
[0117]1 g of 5-(5-fluoro-2-oxo-1,2-dihydro-indol-3Z-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-(carbonyl-1-imidazole), prepared as above, was added to 10 g of 1-methyl-2-pyrrolidone and 0.5 g of diethylendiamine under stirring and the mixture was left for one day...
example 3
Conversion of Sunitinib to Sunitinib Malate (According to Example 1, Preparation A of U.S. publication No. 2003 / 0069298)
[0120]Preparation of the Anhydrous Crystal Form I of the L-Malice Acid Salt of N-[2-(Diethylamino) ethyl]-5-[(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide.
Preparation A:
[0121]N-[2-(Diethylamino)ethyl]-5-[(5-fluoro-1,2-dihydro-2-1-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (130 mg, 0.326 mMol) was added to 20 mL methanol, and the mixture was stirred. L-malic acid (47.2 mg, 0.352 mMol) was added, resulting in rapid dissolution of all the solids. The methanol was removed under reduced pressure to produce a poorly crystalline orange solid. Acetonitrile (5 mL) was added, and the slurry was stirred and heated for about 10 minutes. Stirring was continued while the slurry was allowed to cool to room temperature. The crystals were filtered and dried, resulting in 149 mg of solids (86% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com