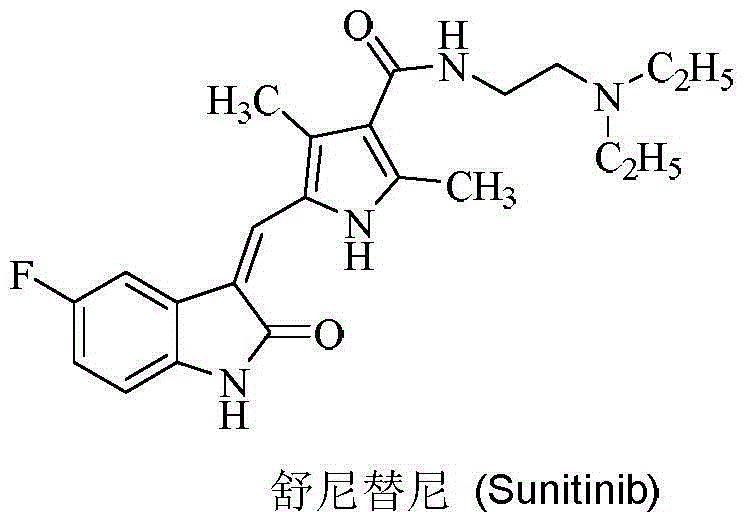
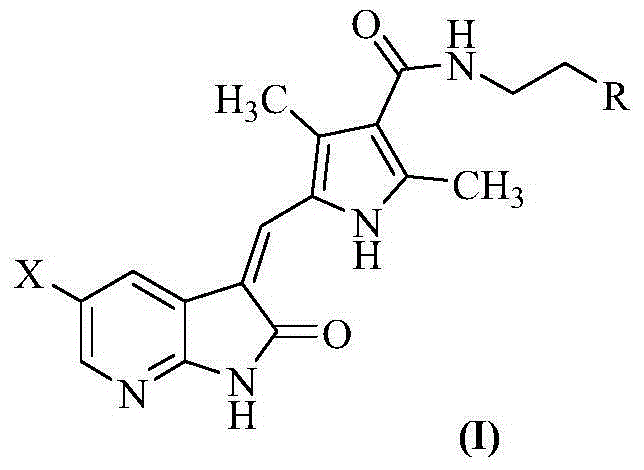
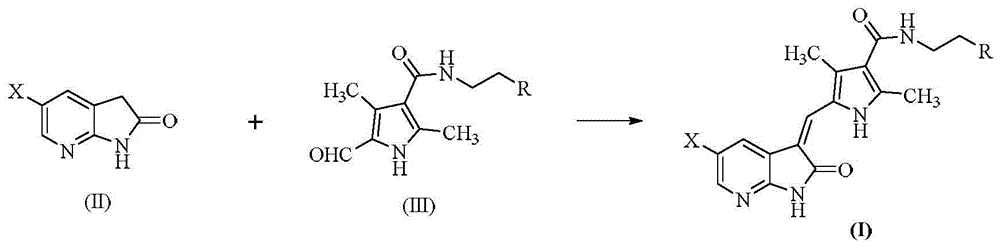
7-azaindolinyl-2-one compounds and preparation method thereof
A compound and drug technology applied in the field of medicinal chemistry to achieve superior anti-tumor activity, high drug safety, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 (Z)-N-[2-(dimethylamino)ethyl]-5-(5-fluoro-2-oxo-1,2-dihydro-3H-pyrrole[2,3-b ]pyridin-3-ylmethylene)-2,4-dimethyl-1H-pyrrole-3-carboxamide
[0050] 2,4-Dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid (167mg, 1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (230mg, 1.2mmol), a mixture of 1-hydroxybenzotriazole (162mg, 1.2mmol) and N,N-dimethylformamide (15mL) was stirred at 0-4°C for 20min, and N,N- Dimethylethylenediamine (0.26mL, 2mmol) was reacted at the same temperature for 30min, then stirred at room temperature for 12h. Dilute with distilled water, adjust the pH to 9 with saturated sodium carbonate solution, extract 15 mL x 3 times with dichloromethane containing 10% methanol, wash once with water and once with saturated brine, and dry over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated under reduced pressure to obtain a red oily liquid (59% yield).
[0051] The above oily liquid and 5-fluoro-7-azai...
Embodiment 2
[0059] Example 2 (Z)-N-{2-[methyl (ethyl) amino] ethyl}-5-(5-fluoro-2-oxo-1,2-dihydro-3H-pyrrole [2 ,3-b]pyridin-3-ylmethylene)-2,4-dimethyl-1H-pyrrole-3-carboxamide
[0060] The same as the preparation method of the compound in Example 1, 2,4-dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid first undergoes a condensation reaction with N,N-methylethylethylenediamine, and then reacts with 5-Fluoro-7-azaindol-2-one was condensed to give an orange solid (yield: 50%).
[0061] 1H NMR (400MHz, DMSO-d6) δ: 13.50(s, 1H), 11.52(s, 1H), 8.19(dd, J=9.2, 2.7Hz, 1H), 7.98(dd, J=2.7, 1.7Hz, 1H),7.81(s,1H),7.48(t,J=5.6Hz,1H),3.28(q,J=6.4Hz,2H),2.57-2.51(m,4H),2.46(s,3H), 2.43(s,3H),2.20(s,3H),0.97(t,J=7.1Hz,3H).
[0062] MS-ESI(m / z):386.63(M+H) + .
[0063] HRMS-ESI(m / z): Calcd.for C 20 h 24 o 2 N 5 F(M+H)+: 386.20126; Found: 386.20113.
Embodiment 3
[0064] Example 3 (Z)-N-[2-(diethylamino)ethyl]-5-(5-chloro-2-oxo-1,2-dihydro-3H-pyrrole[2,3-b ]pyridin-3-ylmethylene)-2,4-dimethyl-1H-pyrrole-3-carboxamide
[0065] Same as the preparation method of the compound in Example 1, 2,4-dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid is first condensed with N,N-diethylethylenediamine, and then reacted with 5 - Chloro-7-azaindol-2-one was condensed to give a yellow solid (yield: 52%), mp: 225-226°C.
[0066] 1H NMR (400MHz, DMSO-d6) δ: 13.45(s, 1H), 11.61(s, 1H), 8.35(d, J=2.2Hz, 1H), 8.02(d, J=2.2Hz, 1H), 7.84 (s,1H),7.48(t,J=5.2Hz,1H),3.28(q,J=6.2Hz,2H),2.55–2.50(m,6H),2.46(s,3H),2.43(s, 3H), 0.98(t, J=7.1Hz, 6H).
[0067] MS-ESI(m / z):416.31(M+H) + .
[0068] HRMS-ESI(m / z): Calcd.for C 21 h 27 o 2 N 5 Cl(M+H) + :416.18478; Found: 416.18492.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com