New crystal form of tini-type medicines
A technology of crystal form and Ni crystal, applied in the field of new crystal forms of tinib drugs and their preparation, can solve the problems of difficult industrialized large-scale production and obtaining high-purity products, moisture absorption, thermodynamic instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
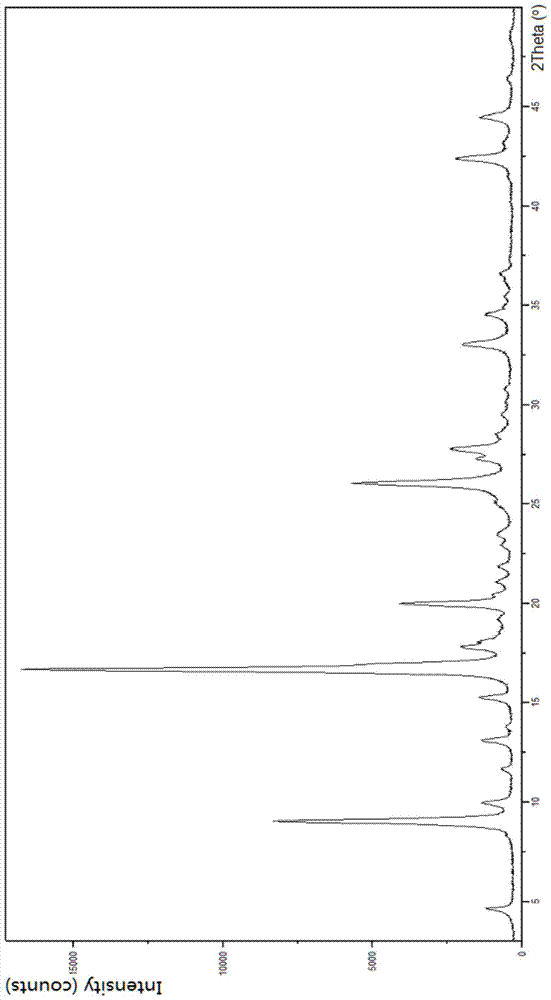
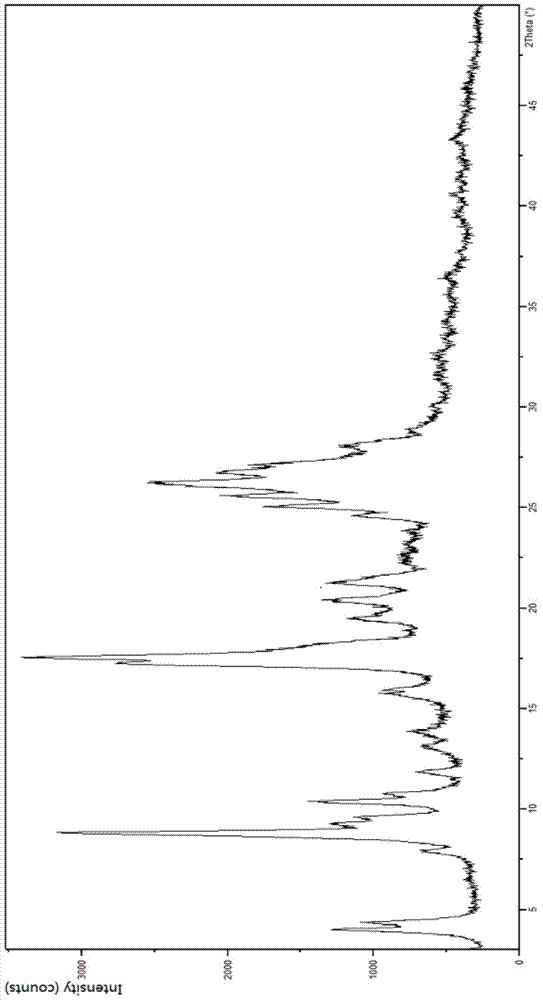
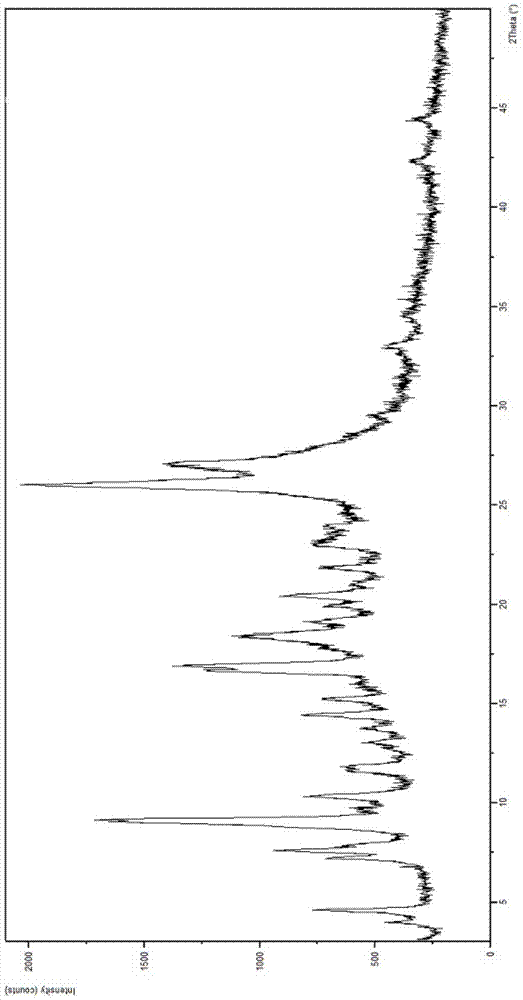
[0023] In the first aspect, the present invention provides substantially pure crystal forms B and C of sunitinib, which are stable and non-hygroscopic, and are very beneficial to industrial operation and production.
[0024] The substantially pure crystal form B of sunitinib has the following characteristics: in its X-ray powder diffraction pattern, there is a peak at a position of about 8.76 degrees in 2θ;
[0025] In some embodiments, its X-ray powder diffraction pattern has peaks at one or more positions at about 8.76, 17.51 and 26.11 degrees 2Θ;
[0026] In some embodiments, its X-ray powder diffraction pattern has peaks at one or more positions at about 8.76, 17.20, 17.51 and 26.11 degrees 2Θ;
[0027] In some embodiments, its X-ray powder diffraction pattern has peaks at one or more of positions at about 3.96, 8.76, 17.51, 21.28, 26.11 and 28.04 degrees 2Θ;
[0028] In some embodiments, its X-ray powder diffraction pattern is about 3.96, 8.76, 9.25, 9.56, 10.34, 15....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com