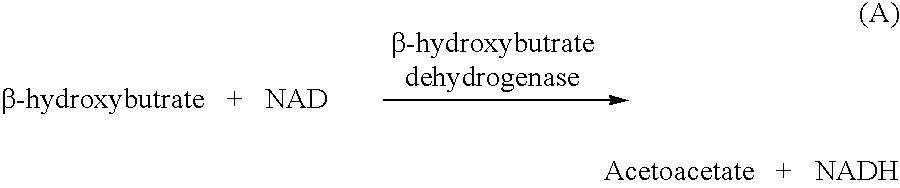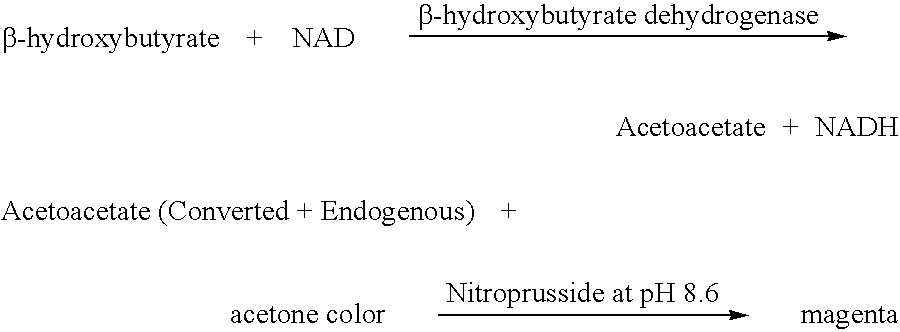Method and test strips for the measurement of fat loss during weight loss programs
a weight loss program and fat loss technology, applied in the field of method and test strips for the measurement of fat loss during weight loss programs, can solve the problems of not being able to measure, body fat must be reduced, and people who experience such weight loss gain back
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1b
A Method and Device to Measure Total Ketone Bodies as One Step with Increased Sensitivity
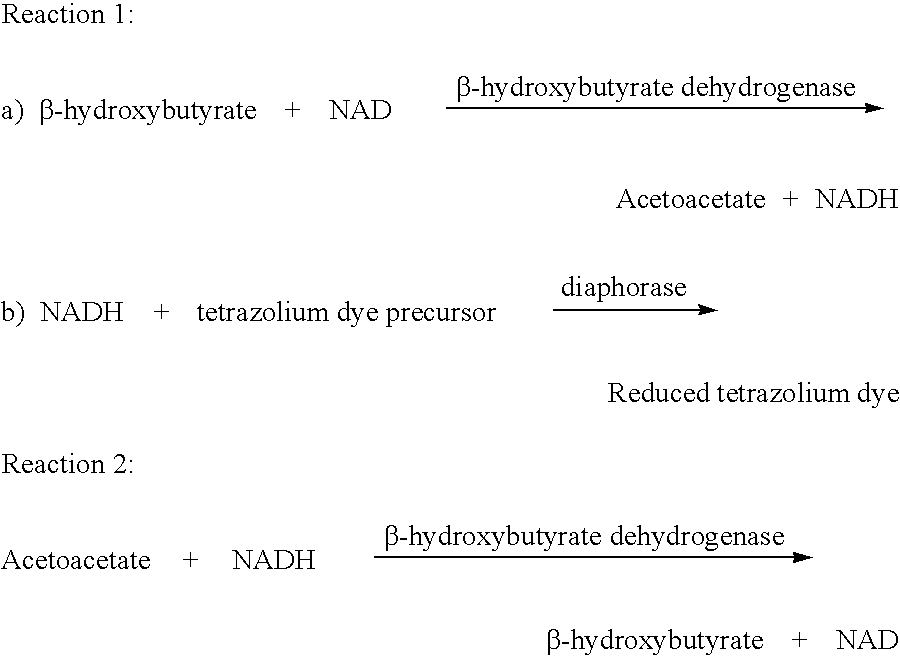
[0053] The formulation contains:
2 Tris Buffer, pH 8.6 0.1M .beta.-hydroxybutyrate dehydrogenase 300 U / mL NAD 3% Sodium Nitroprusside 5% Magnesium sulfate heptahydrate 30% Diaphorase 100 U / mL NBT 2 mM
[0054] The filter paper such as Whatman-54 is dipped in the above formulation and is dried in the oven at 45.degree. C. for 20 minutes. The strips are made by sticking a 1 / 4" of layer of said paper on the bottom of the polystyrene card which is 12" long and 3" high with the help of double adhesive tape. The card is cut lengthwise into 48 strips of 1 / 4".times.3" high strips. These strips are more sensitive in measurement of Total Ketone Bodies (TKB) than those shown in Example 1.
[0055] Similarly to example 1, this formulation contains .beta.-hydroxybutyrate dehydrogenase enzyme (HBD) and NAD which at pH 8.6 converts .beta.-hydroxybutyrate to acetoacetate and NADH on an equimolar basis (Reaction 4) and...
example # 2
EXAMPLE #2
A Method and Device to Measure .beta.-hydroxybutyrate and Acetoacetate Simultaneously in a "Cyclic" Fashion.
[0057] The formulation contains .beta.-hydroxybutyrate dehydrogenase, NAD, NBT and diaphorase at pH 8.0.
[0058] To make the strips, the following ingredients were first mixed:
3 Tris-HCl, pH 8.0 0.1M .beta.-hydroxybutyrate dehydrogenase 100 U / mL (about 4 U / strip) NAD 3% NBT 0.2% Diaphorase 10 U / mL Magnesium chloride 0.1% Surfonyl (a surfactant) 0.06%
[0059] Whatman-54 filter paper was immersed in the above formulation, removed and dried in the oven at 45.degree. C. for 20 minutes. The strips were made by sticking a 1 / 4" layer of said dried paper on the bottom of a polystyrene card of 12" by 3" dimension with the help of double adhesive tape. The card was cut lengthwise into 48 strips of 1 / 4".times.3" strips. These strips were used for testing human bodily fluids. These strips, which measure .beta.-hydroxybutyrate plus acetoacetate are referred to as "HB&AA", and their u...
example # 3
EXAMPLE #3
A Method and Device to Measure .beta.-hydroxybutyrate Alone in Serum (Blood) Samples Obtained from Weight Loss Program that Uses Normal Concentration of .beta.-HBD, Similar to the Device Available Commercially as KetoSite.RTM. from GDS Technology, Inc.
[0060] The formulation contains a normal level of .beta.-hydroxybutyrate dehydrogenase according to the prior art (0.2-5.0 U / mL), NAD, NBT and diaphorase at pH 8.6.
4 .beta.-hydroxybutyrate dehydrogenase (Pseudomonas) 15 U / mL (about 0.2 U per strip) NAD 3% NBT 0.2% Diaphorase 30 U / mL Magnesium chloride 0.1% Surfonyl 0.05% Tris-HCl, (Buffer) pH 8.6 0.1 M
[0061] Whatman-54 filter paper was immersed in the above formulation, removed and dried in the oven at 45.degree. C. for 20 minutes. The strips were made by sticking a 1 / 4" layer of said dried paper on the bottom of a polystyrene card of 12" long by 3" dimension, with the help of double adhesive tape. The card was cut lengthwise into 48 strips of 1 / 4".times.3" strips. These stri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com