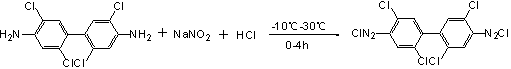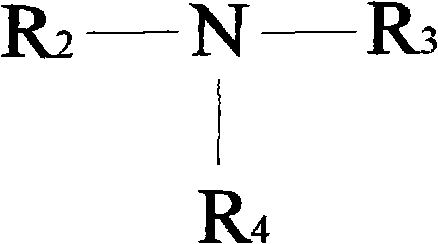Patents
Literature
79 results about "Acetoacetanilide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
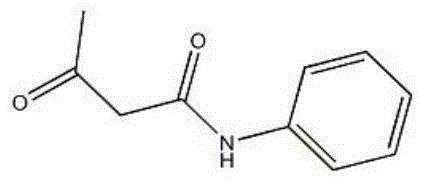
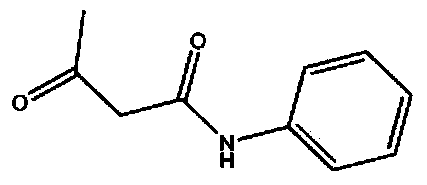
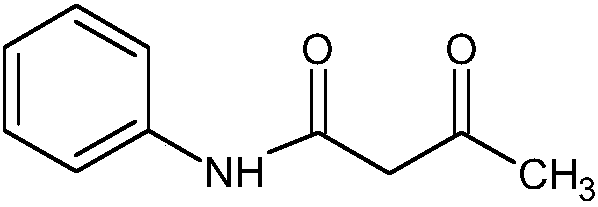
Acetoacetanilide is an organic compound with the formula CH₃C(O)CH₂C(O)NHC₆H₅. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. It and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows.
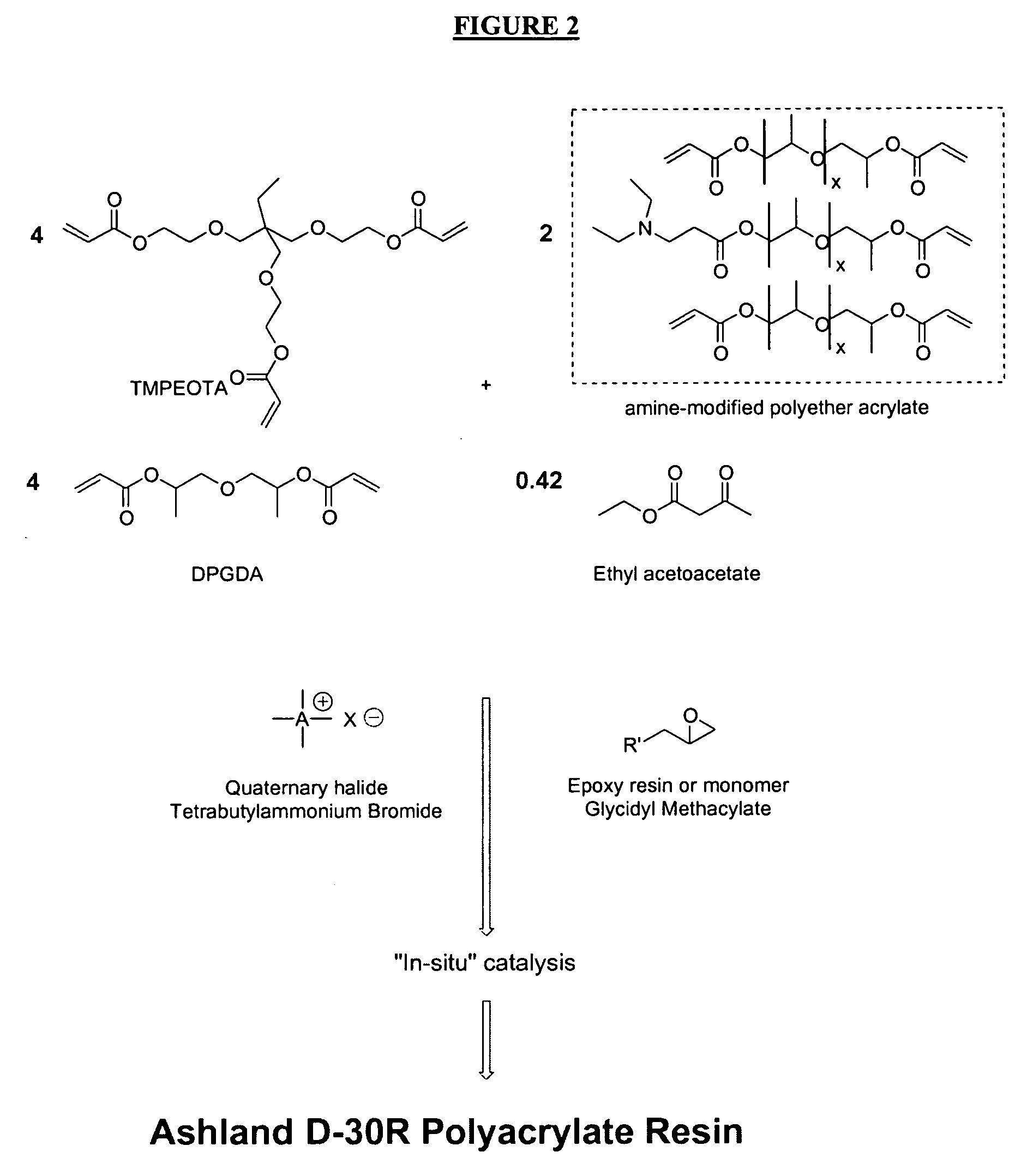
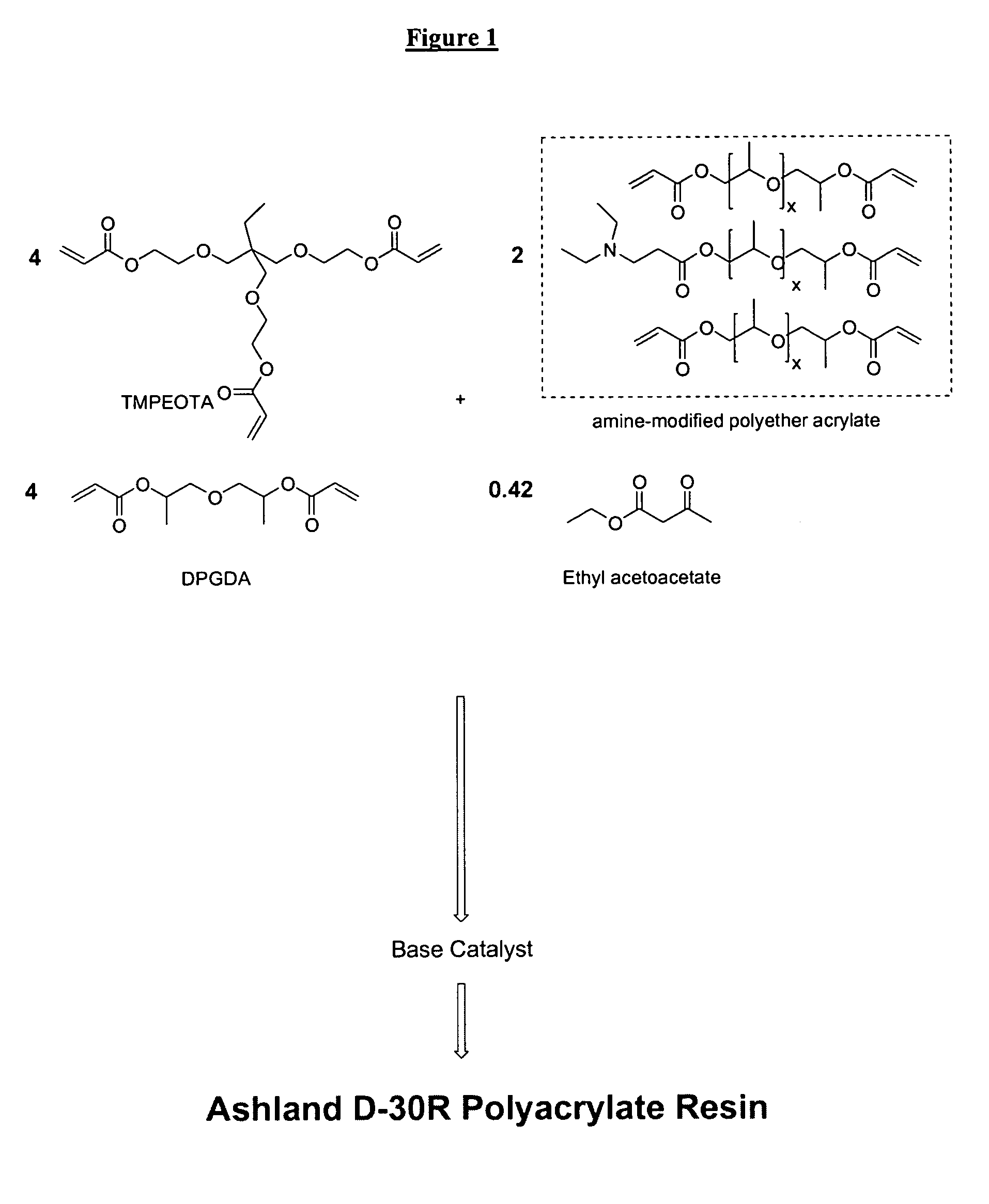
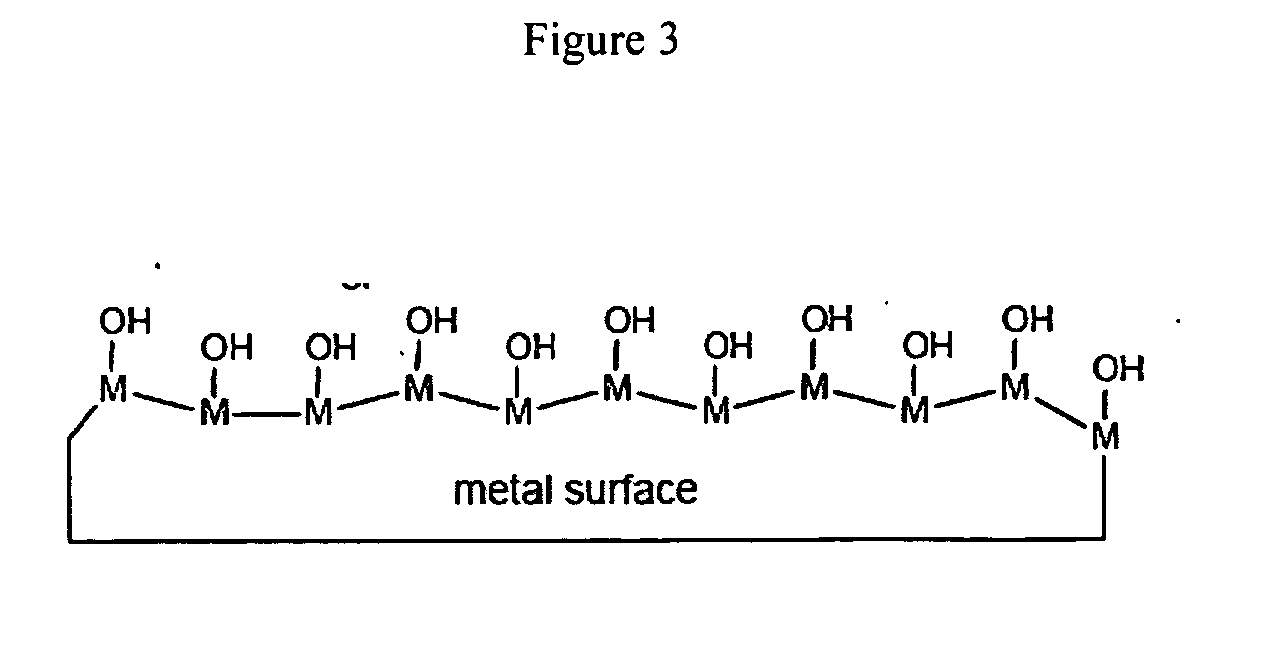
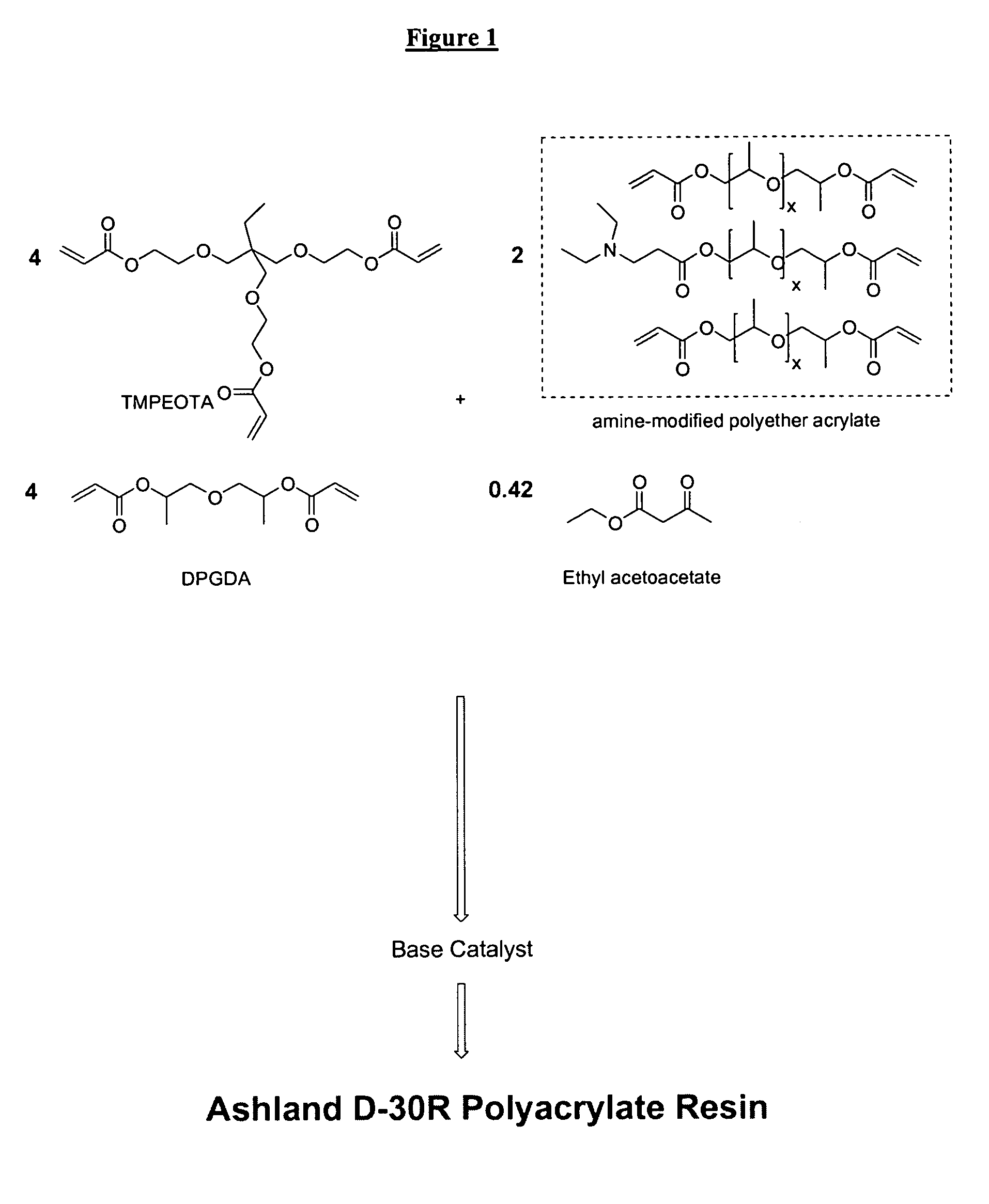
Radiation-curable coatings for metal substrates from multifunctional acrylate oligomers
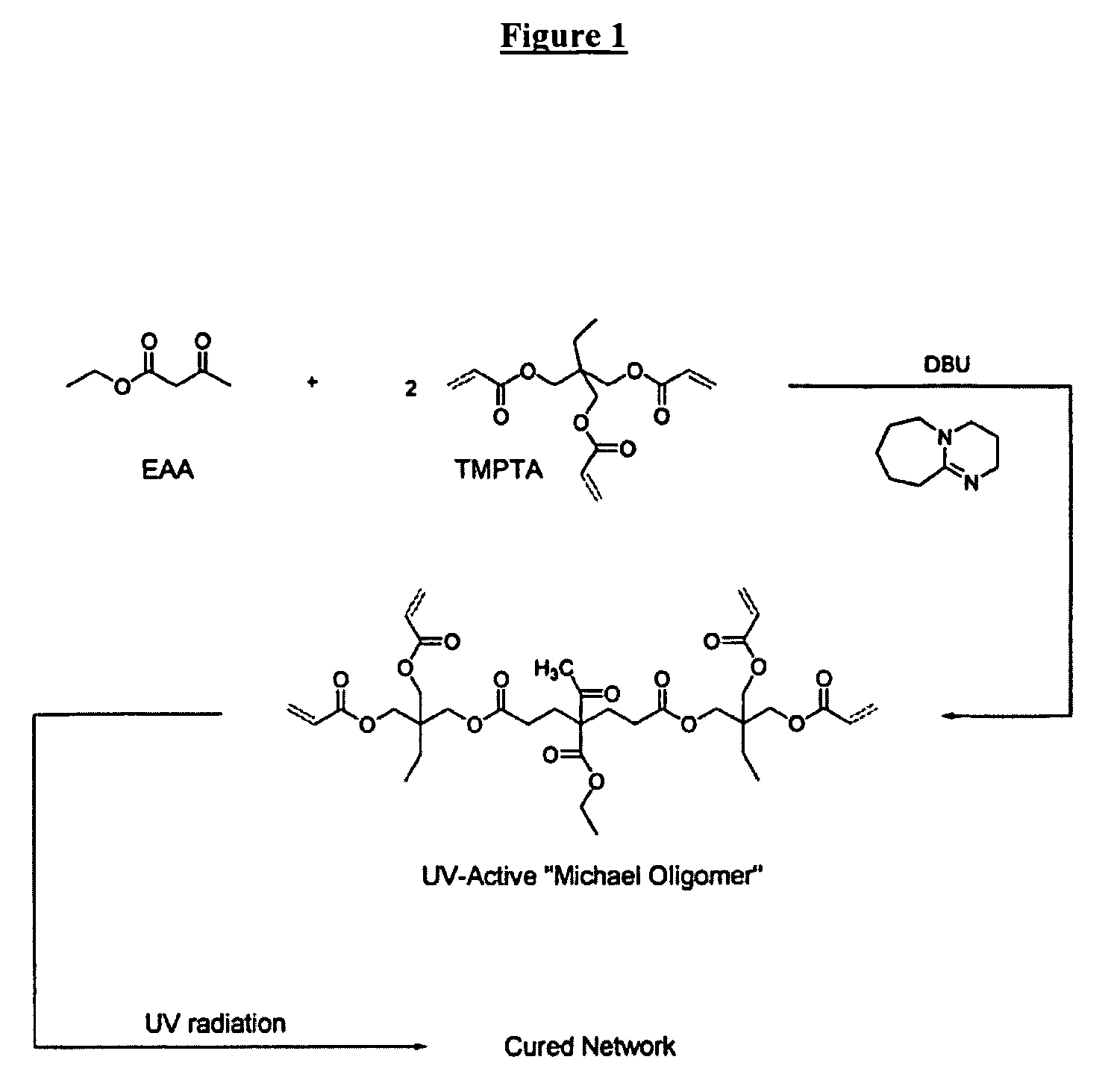
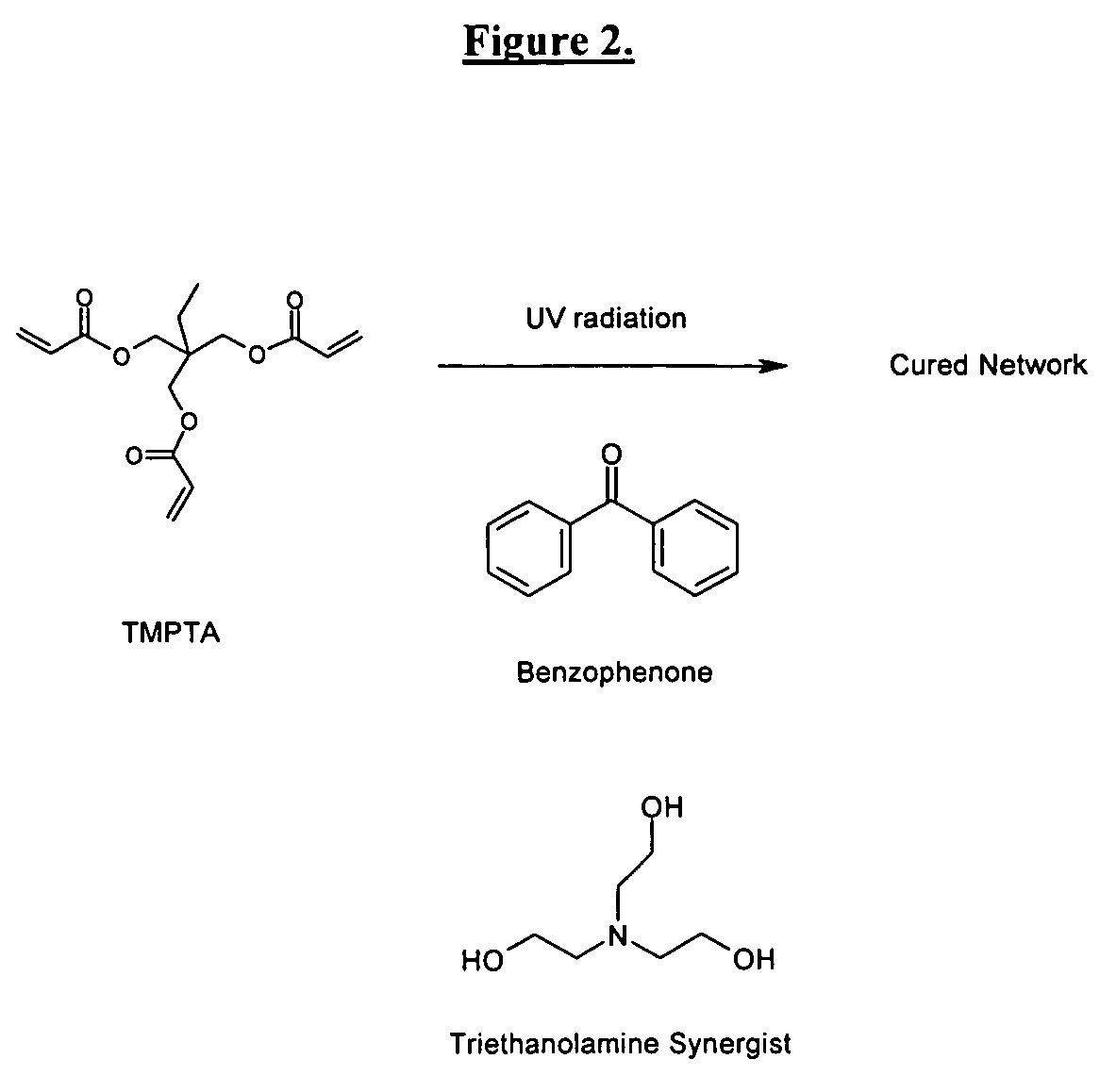
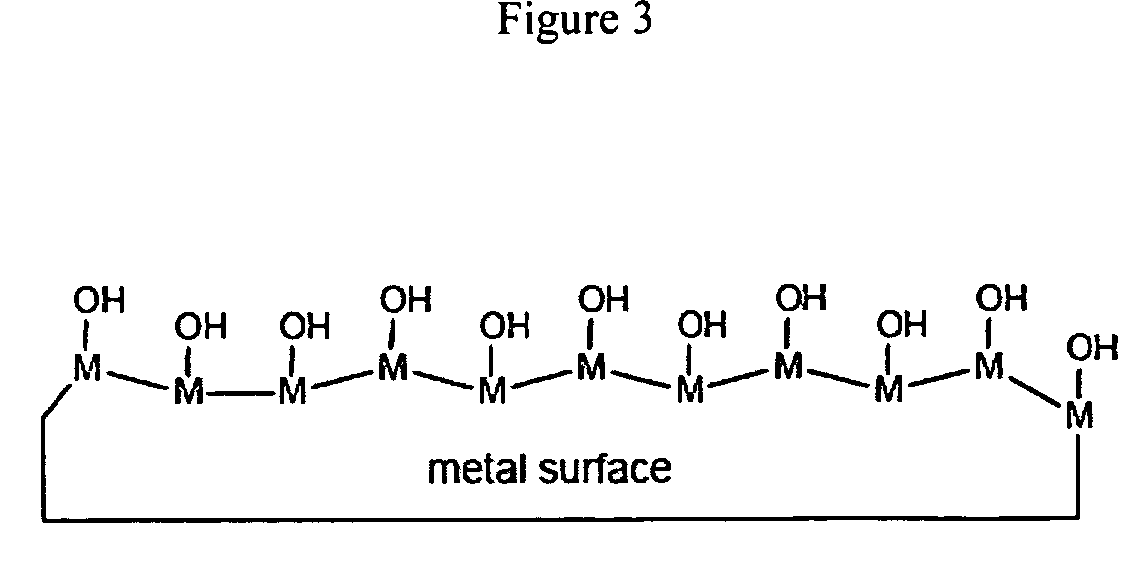
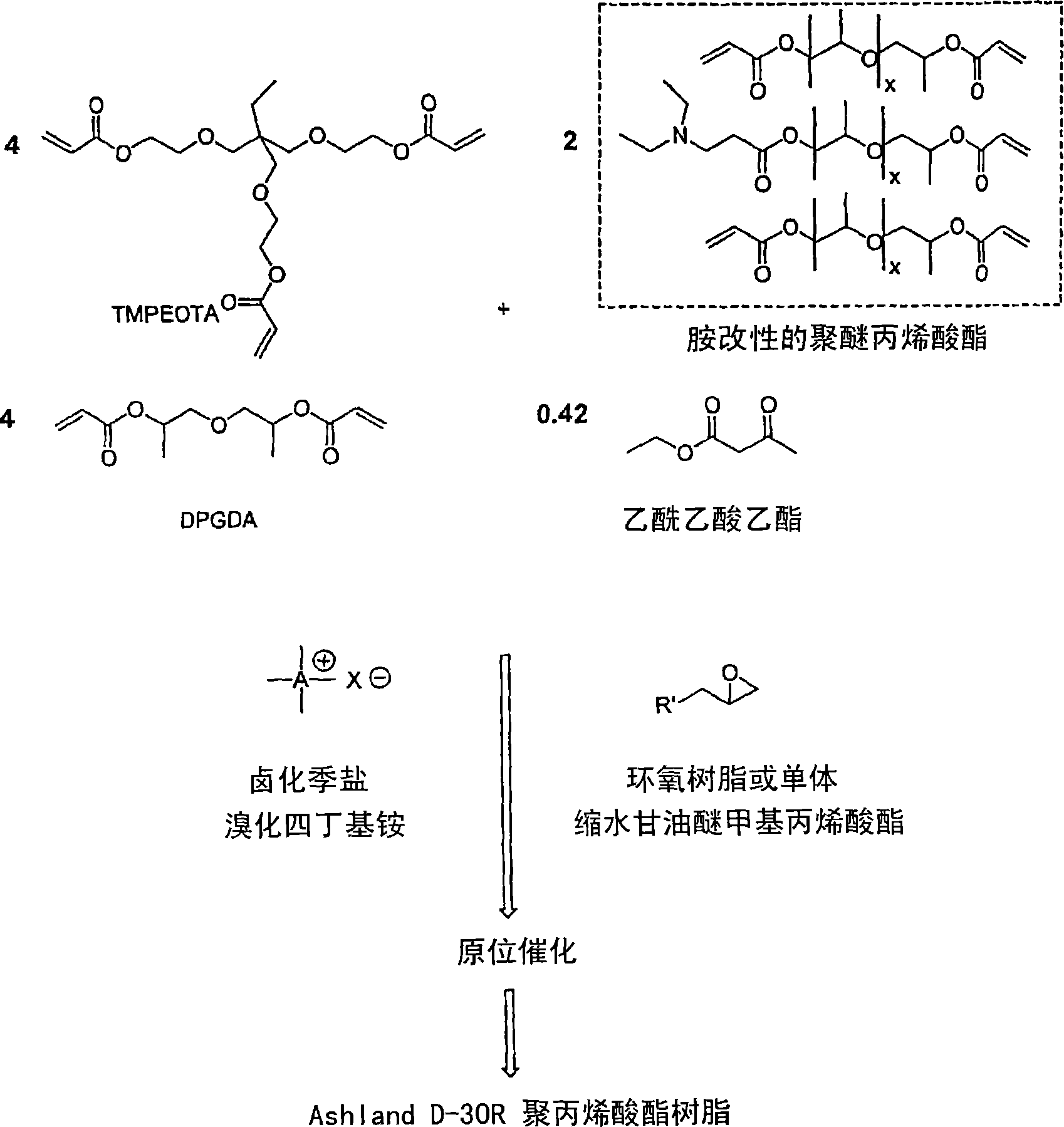
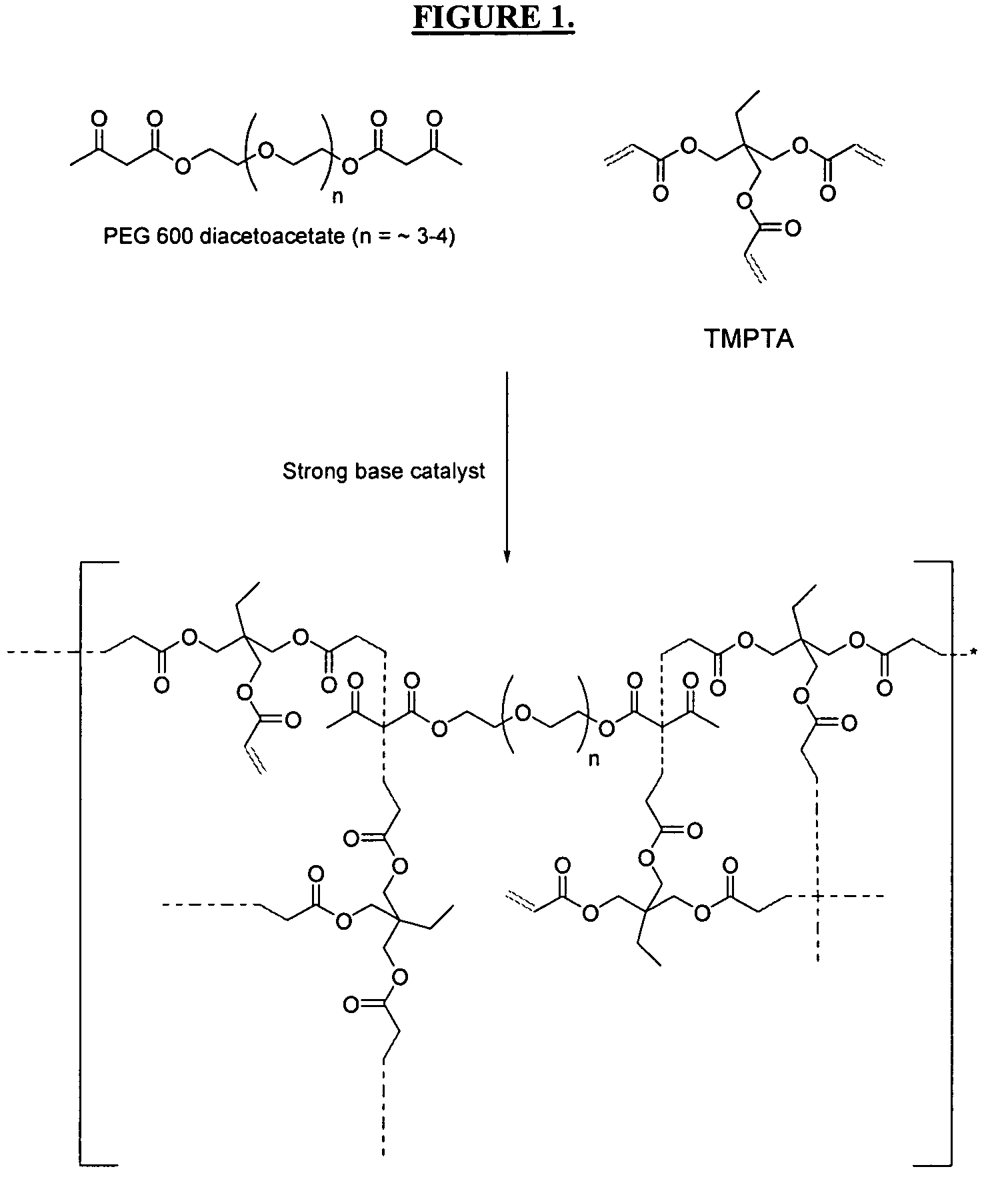
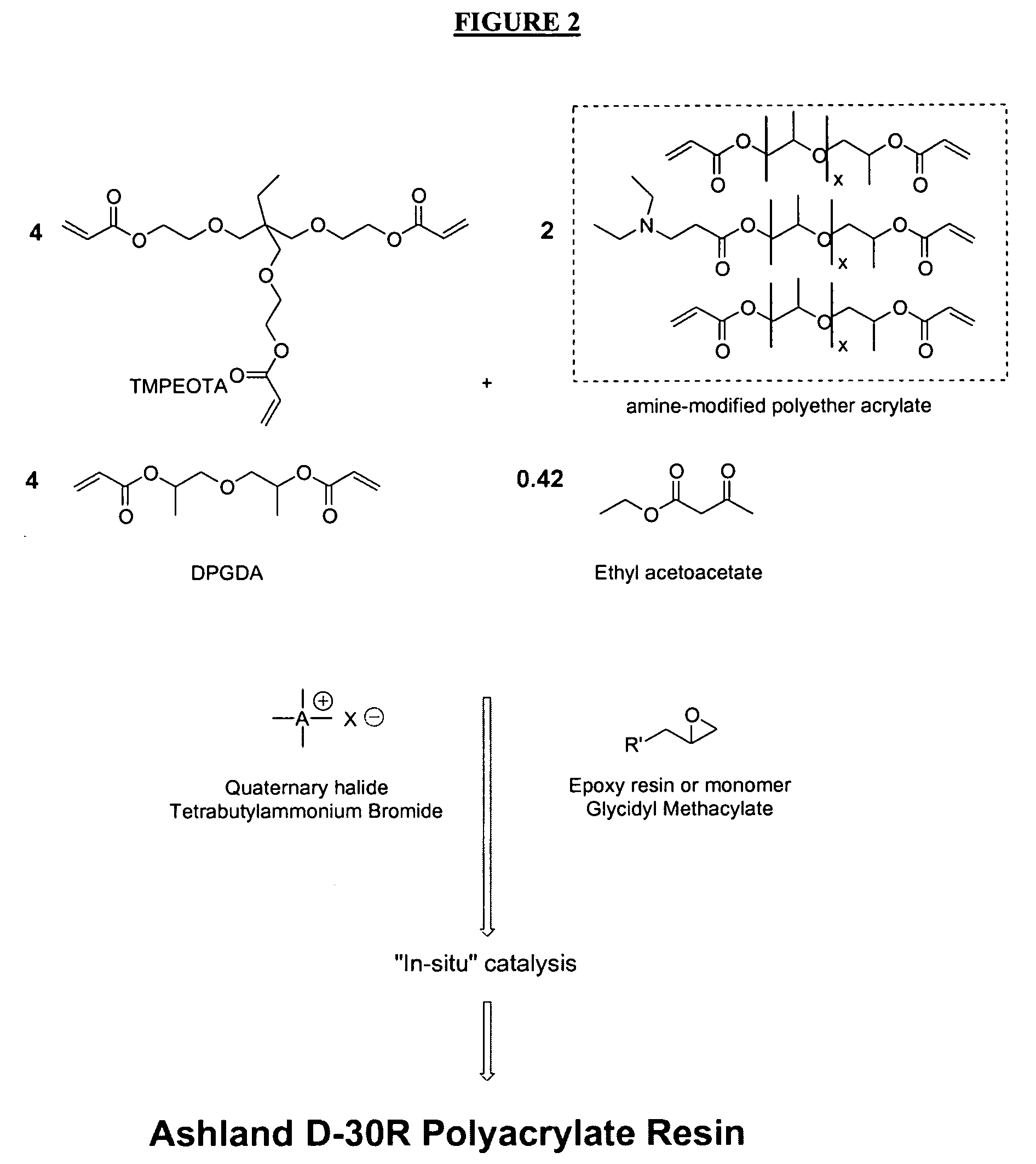
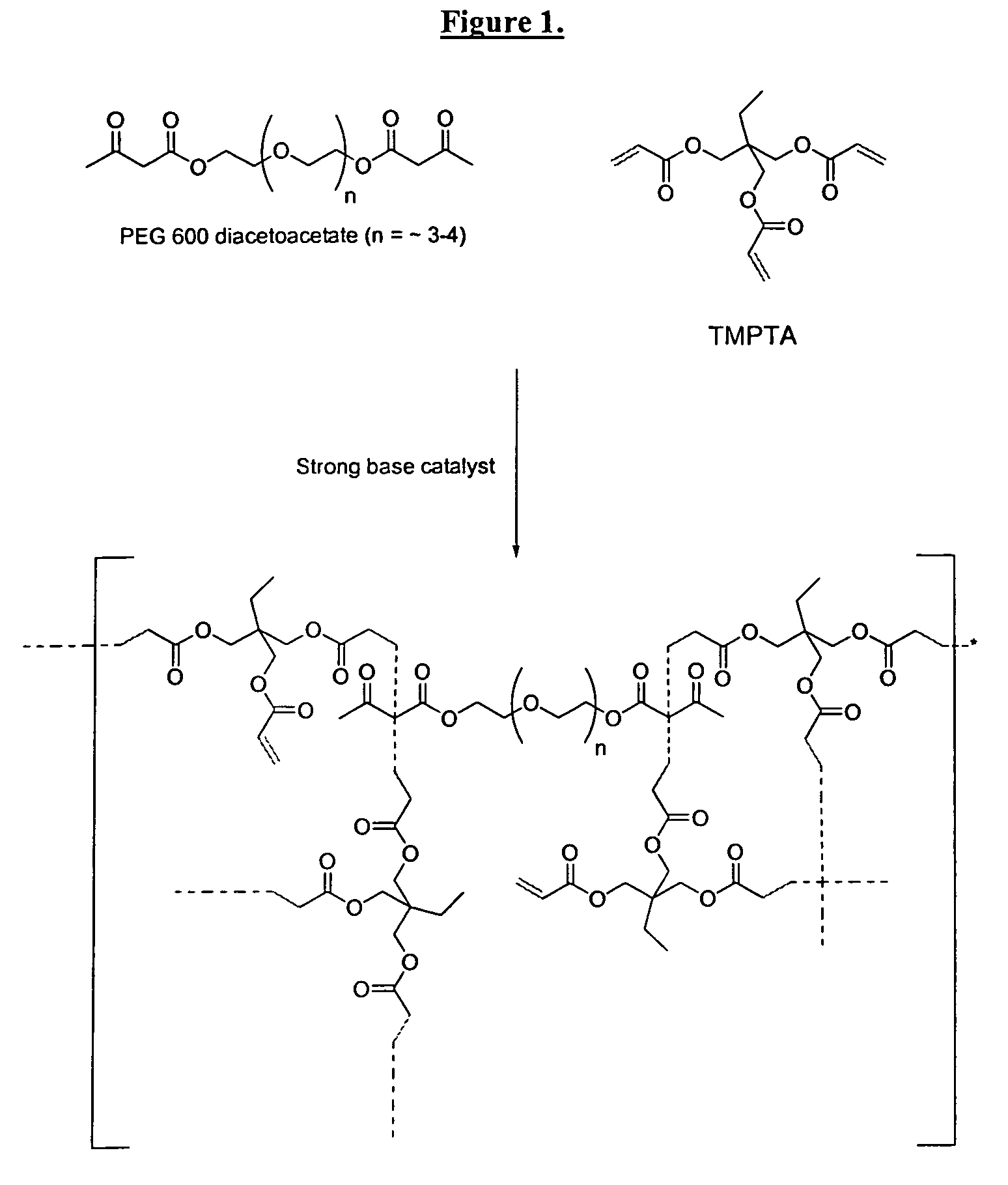
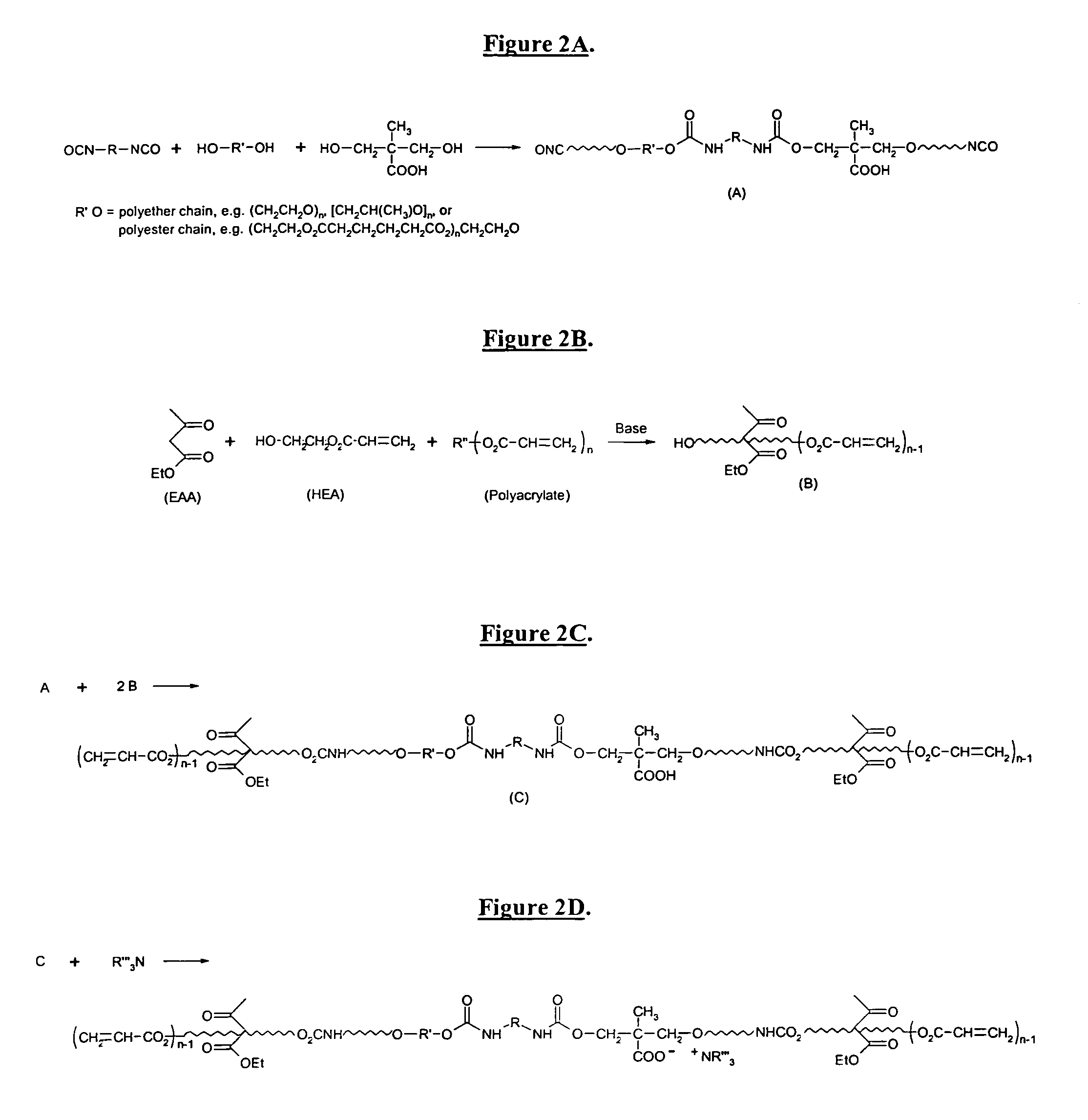
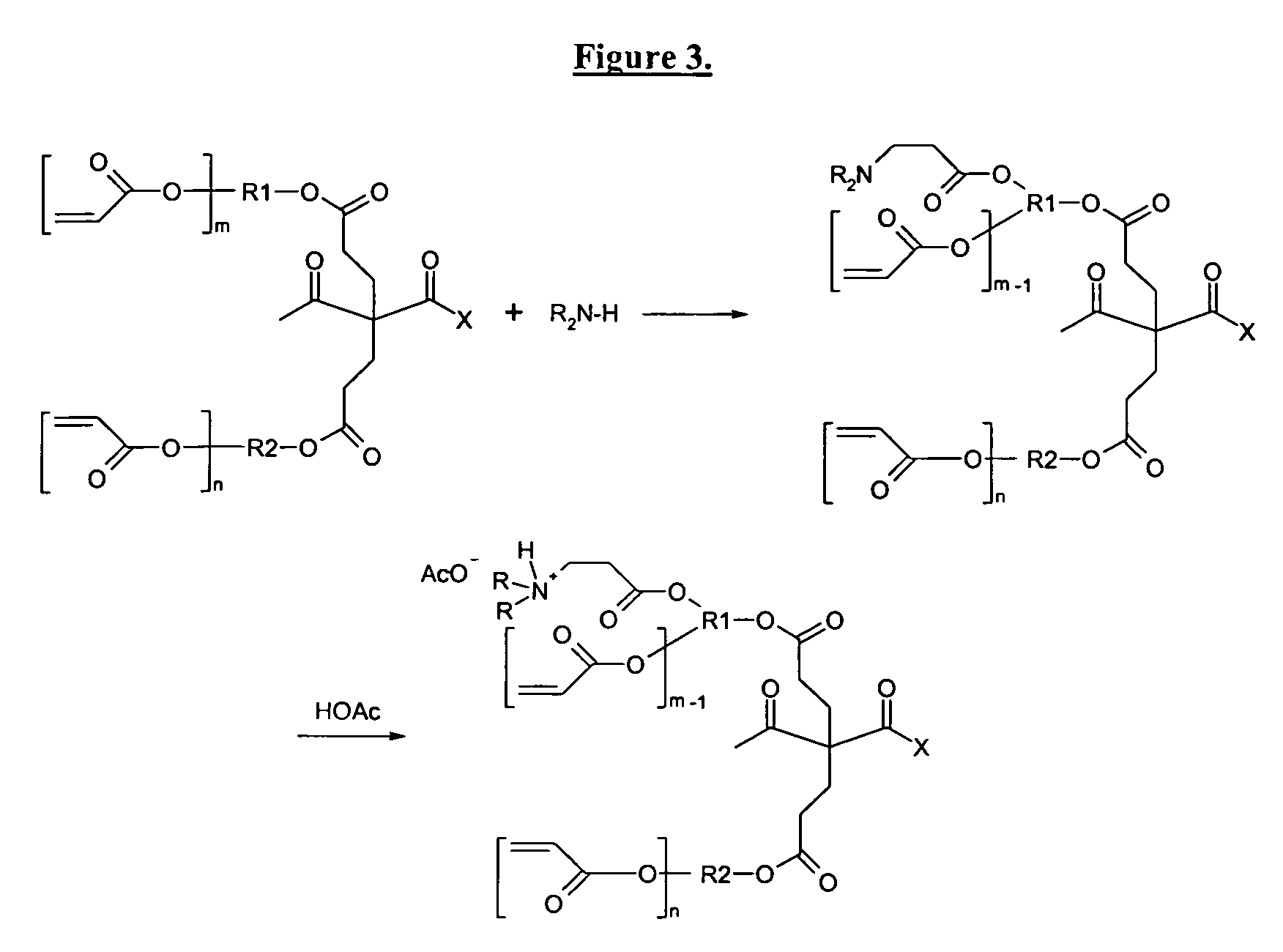
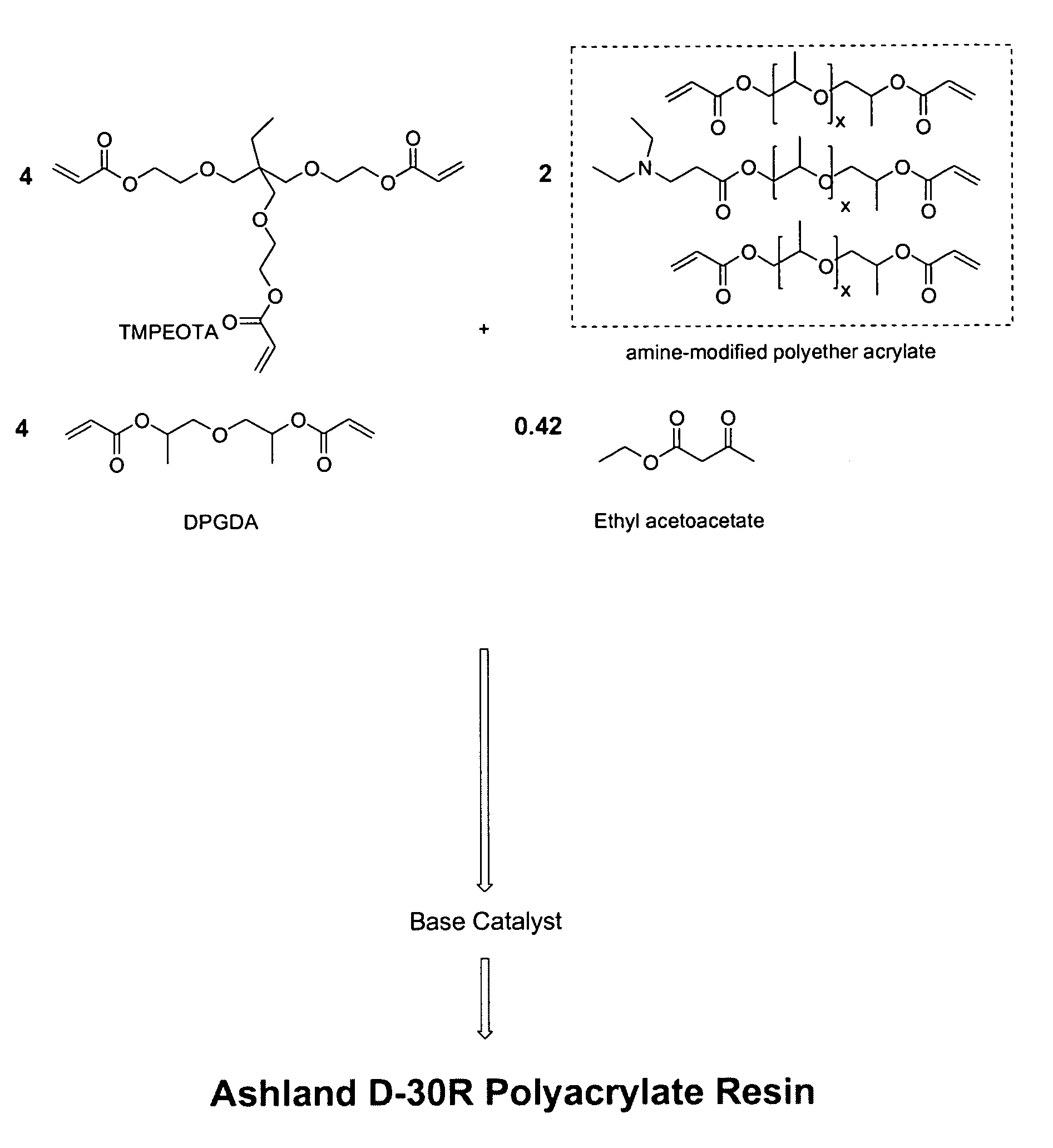
The invention detailed herein comprises a family of radiation-curable coating formulations specifically for metal substrates. These coating formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with ÿ-keto esters (e.g., acetoacetates), ÿ-diketones (e.g., 2, 4-pentanedione), ÿ-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other ÿ-dicarbonyl compounds that can participate in the Michael addition reaction. An essential novelty of these coating resins is that they will cure under standard UV-cure conditions without the addition of traditional photoinitiators. Other materials, both reactive (conventional acrylates) and non-reactive (e.g., solvents) may also be incorporated into the resin oligomers to enhance the coatings properties on metal substrates. These materials include a variety of acrylic monomers and oligomers, primary, secondary and tertiary amines, acid-functional monomers and oligomers, silicones, waxes and elastomers, among others. Coatings based on these novel multifunctional acrylate resins exhibit excellent adhesion and shrinkage control, flexibility, solvent resistance, scratch and mar resistance, impact resistance, color, and durability across a wide range of plastic materials. These coatings may be cured via chemical means, thermally, or by exposure to UV or electron beam radiation.
Owner:ASHLAND LICENSING & INTPROP LLC
Radiation-curable inks for flexographic and screen-printing applications from multifunctional acrylate oligomers
InactiveUS20050080162A1Process economyModifies its propertyLiquid surface applicatorsMixing methodsAcetoacetatesAcetic acid
The present invention relates generally to radiation-curable ink formulations, and particularly, but not by way of limitation, to a family of radiation-curable ink formulations specifically for flexographic and screen printing applications. The inventive ink formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds that can participate in Michael addition reactions.
Owner:ASHLAND LICENSING & INTPROP LLC
Industrial production method for pigment yellow 81
The invention discloses an industrial production method for pigment yellow 81 and belongs to the technical field of fine chemical synthesis. The industrial production method comprises the following steps of: pulping, diazotizing and coupling such materials as 2,2',5,5'-tetrachlorobiphenyl amine hydrochloride, 2,4-dimethyl-N-acetoacetanilide and the like; stirring the materials for 1 hour to 4 hours after the reaction is finished; heating up the reaction liquid to 80 DEG C to 100 DEG C, preserving the heat for reaction for 30 minutes; cooling the reaction liquid to the room temperature, filtering the reaction liquid; and drying the reaction liquid at 70 DEG C to 100 DEG C to obtain the pigment yellow 81. According to the industrial production method disclosed by the invention, the reaction conditions are gentle, the device requirements are low, the reaction time is short; moreover, the obtained product pigment yellow 81has a high yield of more than 90%, good performances, acid resistance of lever four or higher, alkali resistance of level four or high, weather resistance of level six or higher, and heat resistance to 200 DEG C or high, and so the pigment yellow 81 disclosed by the invention can be applied to rubber with high performance requirements and high-level paint.
Owner:CROWN CHEMICAL CORP
Radiation-curable inks for flexographic and screen-printing applications from multifunctional acrylate oligomers
InactiveCN1894291AChange rheologyChange adhesive propertiesInksThin material handlingAcetic acidAcetoacetates
The present invention relates generally to radiation-curable ink formulations, and particularly, but not by way of limitation, to a family of radiation-curable ink formulations specifically for flexographic and screen printing applications. The inventive ink formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with ss-keto esters (e.g., acetoacetates), ss-diketones (e.g., 2, 4-pentanedione), ss-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other ss-dicarbonyl compounds that can participate in Michael addition reactions.
Owner:ASHLAND LICENSING & INTPROP LLC
Preparation method of acetoacetanilide compound
ActiveCN103951582ANot easy to agglomerateReduce energy consumptionOrganic compound preparationCarboxylic acid amides preparationOrganic solventAniline
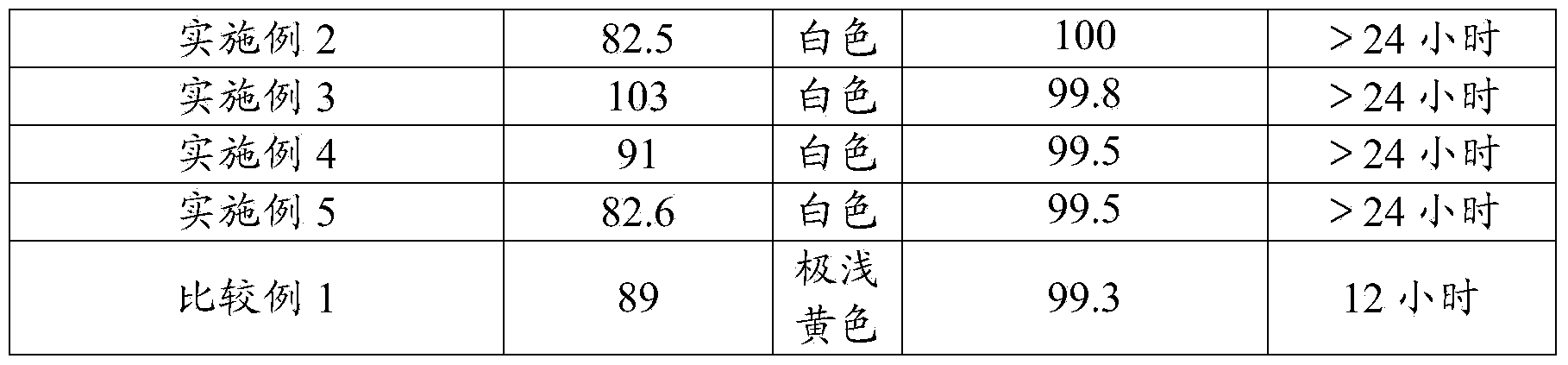
The invention provides a preparation method of an acetoacetanilide compound. The method comprises the steps of performing diacetylation reaction on aniline compounds and diketene in an organic solvent under anaerobic conditions to obtain the acetoacetanilide compound. The acetoacetanilide compound is prepared under the anaerobic conditions; the acetoacetanilide compound prepared by using the method has a relatively high melting point, thereby being beneficial for applications of the acetoacetanilide compound. In addition, the acetoacetanilide compound prepared by using the method provided by the invention is not easy to agglomerate, and the preparation method of the acetoacetanilide compound, provided by the invention, is relatively low in energy consumption. Experimental results show that the melting point of the acetoacetanilide compound prepared by using the method provided by the invention is 82.5-103 DEG C.
Owner:青岛海湾集团有限公司
Radiation-curable lithographic inks from multifunctional acrylate oligomers
The present invention relates generally to radiation-curable ink formulations, and particularly, but not by way of limitation, to a family of radiation-curable ink formulations specifically for lithographic printing applications. The inventive ink formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds that can participate in Michael addition reactions.
Owner:ASHLAND LICENSING & INTPROP LLC
Preparation method for N-acetyl acetanilide
ActiveCN103224455APrevent mutual condensationReduce the amount of waterOrganic compound preparationCarboxylic acid amide separation/purificationBiotechnologyEthenone
The invention relates to a preparation method for N-acetyl acetanilide. The method comprises the following steps of adding N-acetyl acetanilide crystal seeds and an emulsifying agent to deionized water at a temperature of 0-10 DEG C to carry out a reaction; then dropwise adding diketene and aniline simultaneously under the control of a certain temperature; keeping the reaction at the temperature; cooling to a temperature of 0 DEG C; filtering and drying to obtain the N-acetyl acetanilide. The method has the advantages of effectively preventing product caking, improving product appearance and performance, increasing product yield and greatly reducing wastewater quantity.
Owner:NANTONG ACETIC ACID CHEM
Acetoacetanilide wastewater recycling process
InactiveCN105036234AImprove adsorption efficiencyEasy desorption and regenerationWater contaminantsWater/sewage treatment by sorptionDesorptionFiltration
The invention relates to the technical field of industrial wastewater treatment process, in particular to an acetoacetanilide wastewater recycling process. Acetoacetanilide wastewater is firstly subjected to preliminary filtration and then is recycled by means of the adsorption action of resin; afterwards the resin is subjected to desorption treatment, and finally recycling is realized and waste is reduced. The treatment process is simple, operation and management are convenient, a device is simple and small in occupied area, and NDA-150 resin has a property of selectively adsorbing naphthalene type organic matters, is high in adsorption efficiency and easy for desorption regeneration. In a whole wastewater treatment process, any new pollutant is not introduced, and secondary pollution is not generated. The NDA-150 resin is high in acid and alkali resistant capacity, high in mechanical strength and good in temperature resistance and has the service life of at least 4 years.
Owner:JIANGSU TIANCHENG BIOCHEM PROD
Crystallization method of N-acetoacetanilide type compounds
ActiveCN104370767AShort crystallization timeIncrease productivityOrganic compound preparationCarboxylic acid amide separation/purificationAnilineAcetoacetanilide
The invention relates to the field of synthesis of N-acetoacetanilide type compounds, and in particular relates to a crystallization method of N-acetoacetanilide. According to the crystallization method, gradient cooling is performed on materials by introducing a condensation medium into a cooling tube of a reaction kettle. The crystallization method disclosed by the invention is short in crystallization time, simple in operation step, capable of achieving automation and standardization and capable of improving the yield and purity of products, the particle sizes of product crystals are uniform and consistent, the phenomenon of agglomeration is further reduced, and the agglomeration time is greatly prolonged.
Owner:青岛海湾集团有限公司
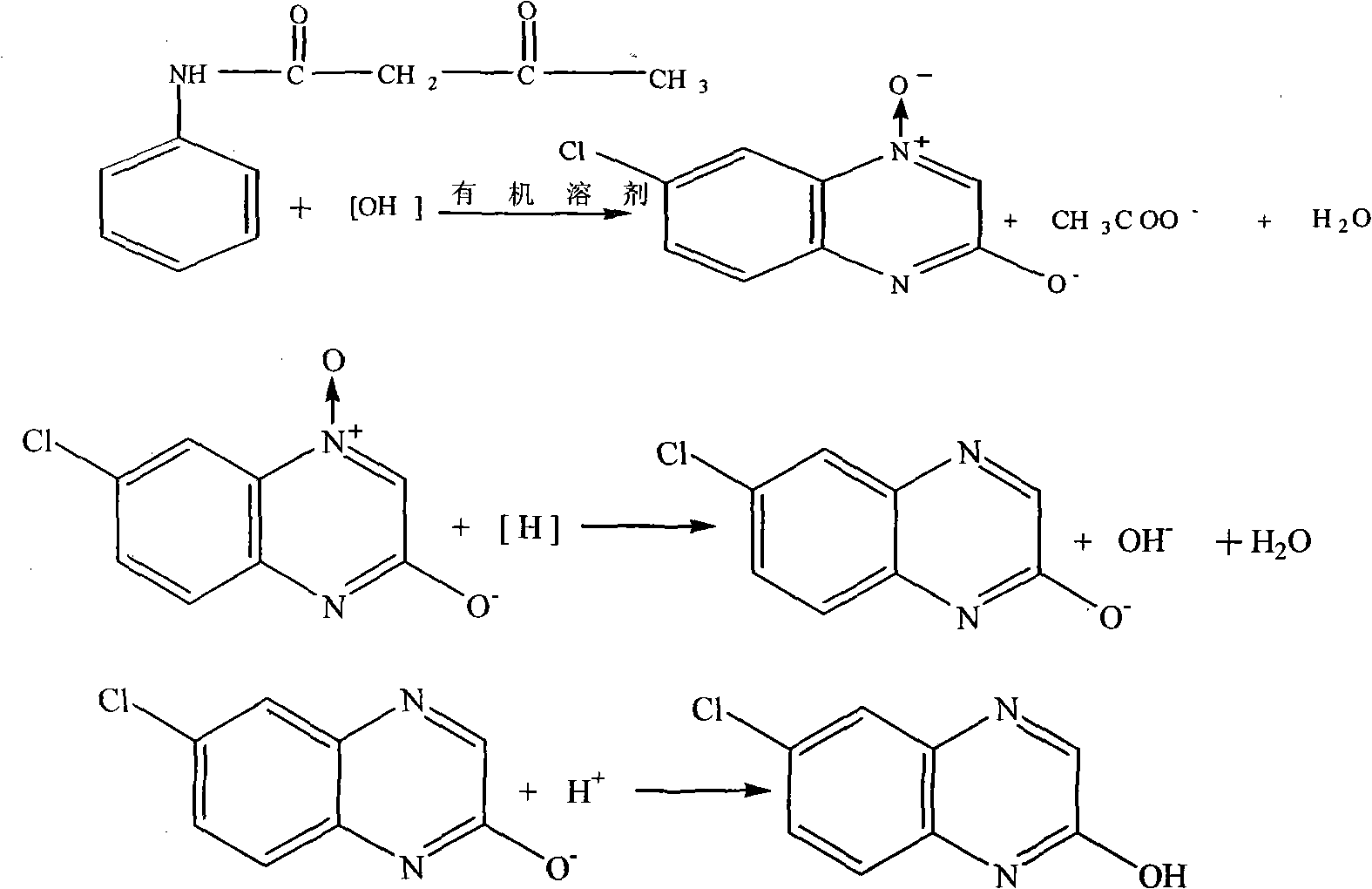
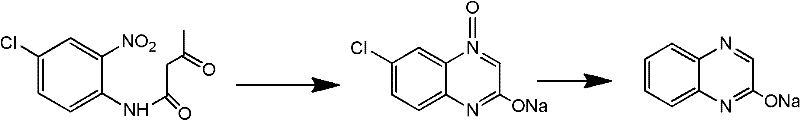
Synthesis method of 2-chloro-6-chloroquinoxaline
ActiveCN101941947AReduce generationReduce pollutionOrganic chemistryOrganic solventSynthesis methods
The invention belongs to the field of organic synthesis, providing a synthesis method in which an organic solvent is used as a synthesis environment of 2-chlorine-6-chloroquinoxaline. The method adopts p-chloro-m-nitroacetoacetanilide as a starting raw material, and a metal salt is obtained through closed loop, reduction and crystallization in the organic solvent under the alkaline condition. The technology is adopted to improve the stability of the whole synthesis reaction. Compared with the traditional technology, the method has the advantages of simple and practicable operation, high yield and content, wherein the use and the recycle of the organic solvent can greatly reduce the generation of waste water, relieve the pollution of environment and reduce production cost.
Owner:JINGBO AGROCHEM TECH CO LTD +1
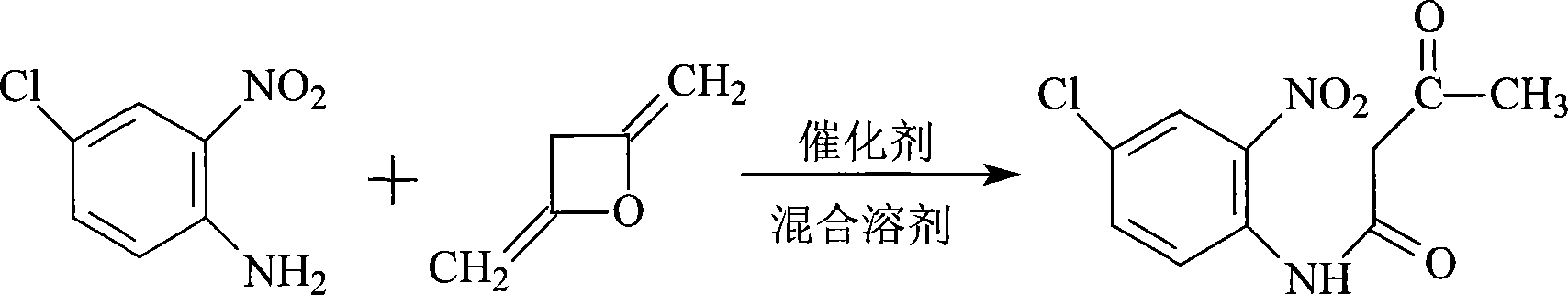
Method for preparing high-purity p-chloro-m-nitroacetoacetanilide
ActiveCN101177404AImprove solubilityHigh purityOrganic compound preparationCarboxylic acid amides preparationEthyl acetateDiketene
The invention discloses a method for preparing high-purity p-chloro-m-nitroacetoacetanilide, in which adopts the technical proposal that p-chloro-o-nitroaniline and catalyst are added in mixed solvent at 15 to 100 DEG C, and then diketene is dripped within 0.5 to 4 hours for reaction for 0.5 to 6 hours to prepare the p-chloro-m-nitroacetoacetanilide, wherein the mixed solvent can be either the solvent composed of two or more materials among water, acetone, tetrahydrofuran, acetonitrile, ethanol, toluene, benzene, isopropyl alcohol, chloroform, dichloromethane, ethyl acetate, butyl acetate and methyl acetate or circulating mother liquor. The circulating mother liquor can be recycled for as many as 10 times and extra catalyst is not needed with the purity of products exceeding 99% and the yield reaching 97%. The invention has the advantages of simple and controllable process, no need of recrystallization and high purity.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
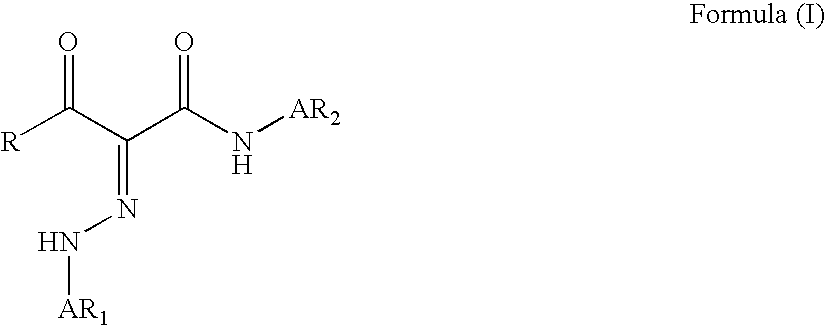
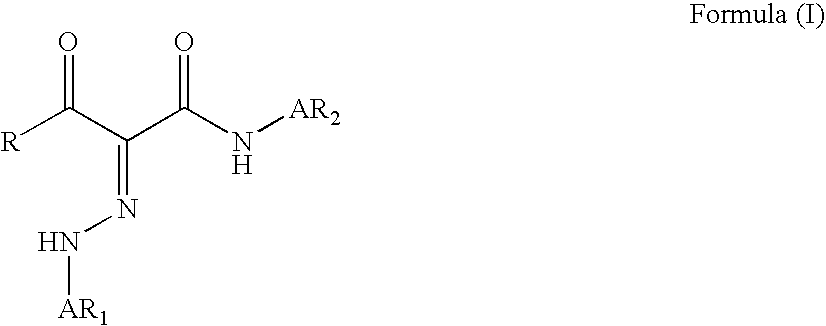
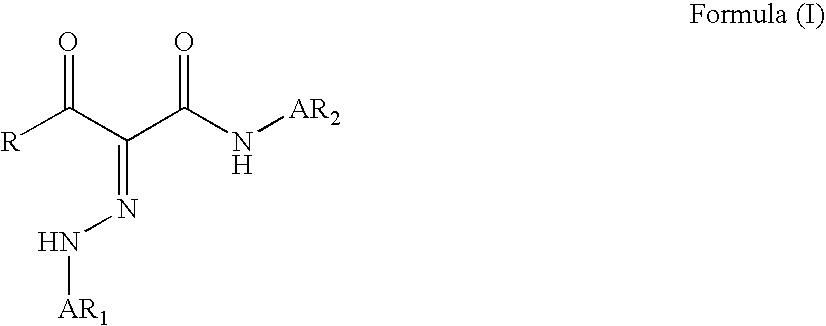
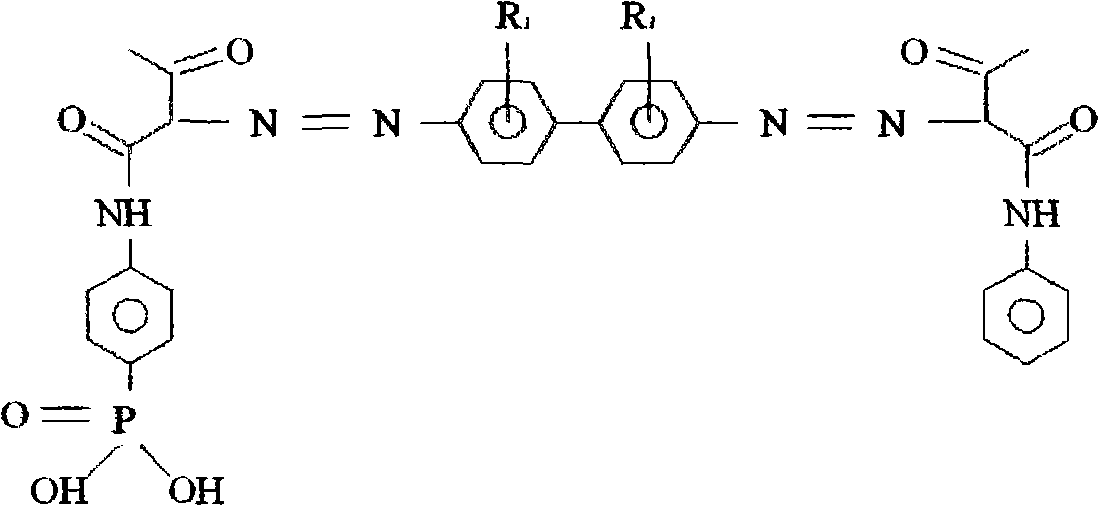
Phenylazo-acetoacetanilide derivatives with a polymerizable functional group and related compounds as monomers for preparing polymeric pigment dispersants for inkjet inks
InactiveUS7812113B2Improve dispersion stabilityHigh optical densityIsocyanic acid derivatives preparationMonoazo dyesAcetoacetanilidePhotochemistry
Owner:AGFA NV
Synthesis and crystallization method of N-acetoacetanilide compound
ActiveCN104356018AShort crystallization timeIncrease productivityOrganic compound preparationCarboxylic acid amide separation/purificationSynthesis methodsCooling pipe
The invention relates to the field of synthesis of N-acetoacetanilide compound, in particular to a synthesis and crystallization method of the N-acetoacetanilide compound. The synthesis method is to cool the material by feeding a condensation medium to a cooling pipe of a reaction kettle in a gradient manner. The crystallization method disclosed by the invention is short in crystallization time, simple in operation steps, capable of implementing automation and standardization, and further capable of increasing the yield and the purity of the product; the particle sizes of the crystals of the product are uniform and consistent; the agglomeration phenomenon can be further reduced; the agglomeration time can be greatly prolonged.
Owner:QINGDAO DOUBLE PEACH SPECIALTY CHEM GRP
Phenylazo-Acetoacetanilide Derivatives with a Polymerizable Functional Group and Related Compounds as Monomers for Preparing Polymeric Pigment Dispersants for Inkjet Inks
InactiveUS20080177016A1Improve dispersion stabilityHigh optical densityIsocyanic acid derivatives preparationMonoazo dyesAcetoacetanilidePhotochemistry
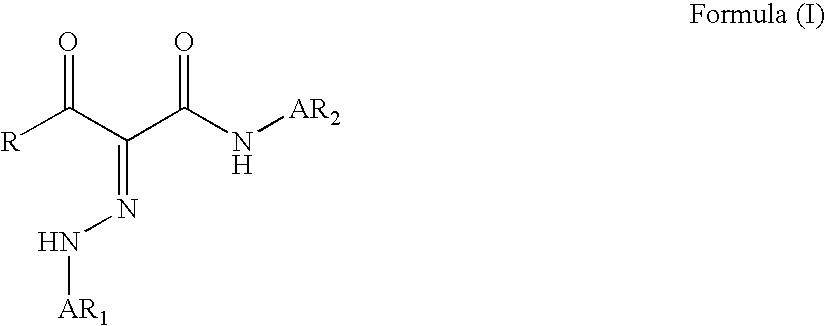
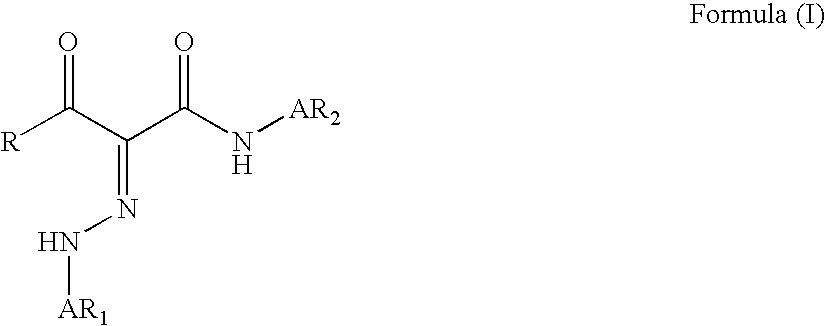
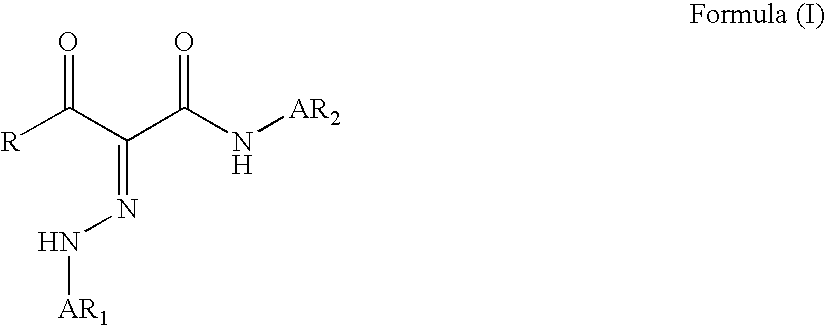
A monomer with a chromophore group represented by Formula (I):whereinAR1 represents a substituted or unsubstituted aromatic group;AR2 represents a substituted or unsubstituted aromatic group or a substituted or unsubstituted alkyl group;and R represents a substituted or unsubstituted alkyl group, with the proviso that one of R, AR1, and AR2 has a substituent with a polymerizable functional group. The monomer can be advantageously used to prepare polymeric dispersants for pigment dispersion, especially inkjet inks.
Owner:AGFA NV
Radiation-curable lithographic inks from multifunctional acrylate oligomers
InactiveUS20050245630A1Process economyModifies its propertyPlaten pressesLayered productsAcetoacetatesKetone
The present invention relates generally to radiation-curable ink formulations, and particularly, but not by way of limitation, to a family of radiation-curable ink formulations specifically for lithographic printing applications. The inventive ink formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds that can participate in Michael addition reactions.
Owner:ASHLAND LICENSING & INTPROP LLC
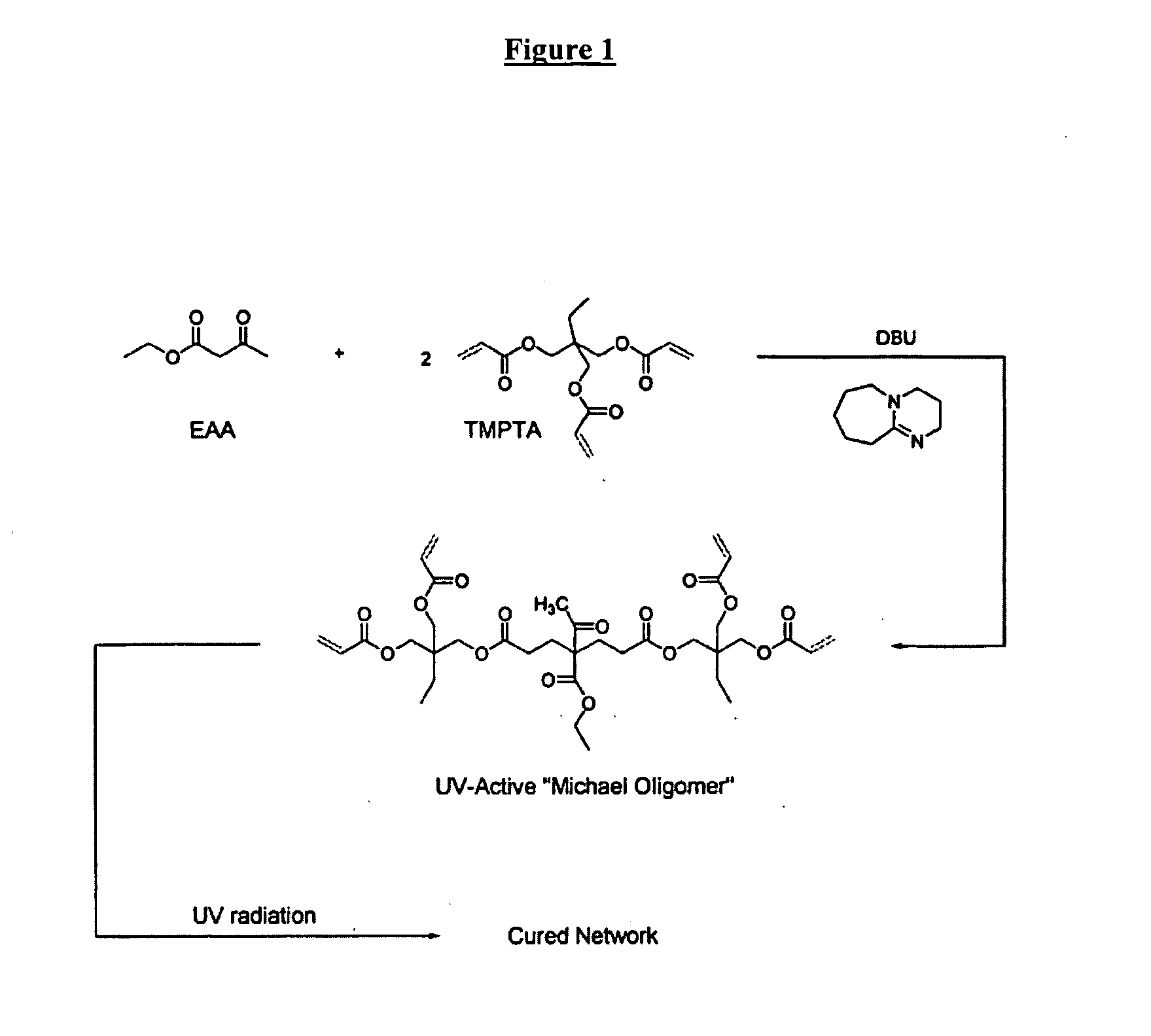
Multifunctional acrylate oligomers as pigment grinding vehicles for radiation-curable ink applications
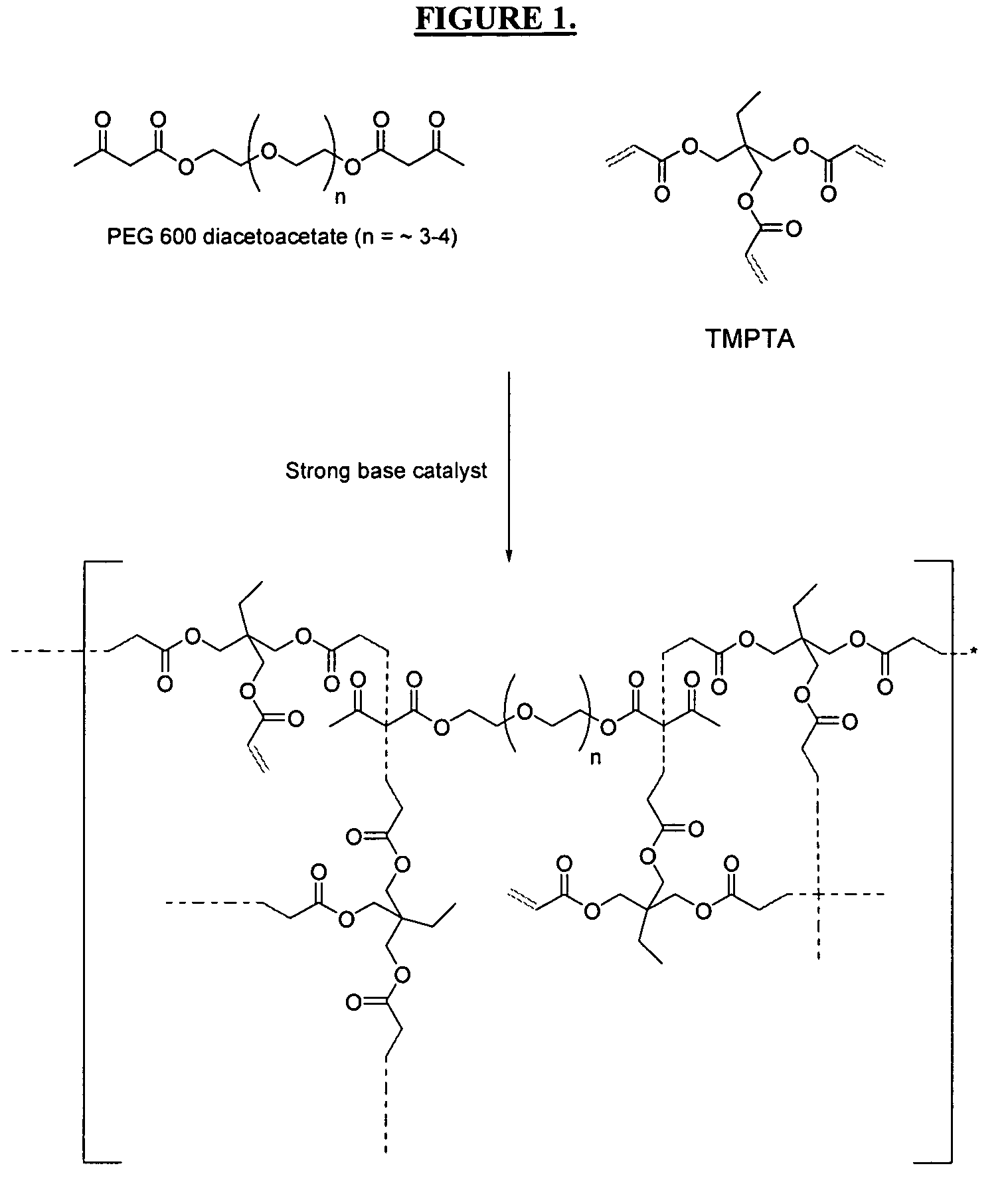
The invention detailed herein comprises a family of multifunctional acrylate oligomers which are useful as pigment grinding vehicles for use in radiation-curable coating formulations. The multifunctional acrylate resins are formed by the reaction of acrylate monomers and oligomers with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds or Michael “donors” that can participate in the Michael addition reaction. These resin vehicles have a built-in chromophore that enables ink formulations made from their dispersions to cure under standard UV-cure conditions with significantly less photoinitiator than commercial formulations. The resins also exhibit excellent pigment wetting characteristics and can be designed to function as a single dispersion vehicle for different pigments and different ink applications such as screen, flexographic, and lithographic printing.
Owner:ASHLAND LICENSING & INTPROP LLC
Radiation-curable coatings for metal substrates from multifunctional acrylate oligomers
The invention detailed herein comprises a family of radiation-curable coating formulations specifically for metal substrates. These coating formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds that can participate in the Michael addition reaction. An essential novelty of these coating resins is that they will cure under standard UV-cure conditions without the addition of traditional photoinitiators. Other materials, both reactive (conventional acrylates) and non-reactive (e.g., solvents) may also be incorporated into the resin oligomers to enhance the coatings properties on metal substrates. These materials include a variety of acrylic monomers and oligomers, primary, secondary and tertiary amines, acid-functional monomers and oligomers, silicones, waxes and elastomers, among others. Coatings based on these novel multifunctional acrylate resins exhibit excellent adhesion and shrinkage control, flexibility, solvent resistance, scratch and mar resistance, impact resistance, color, and durability across a wide range of plastic materials. These coatings may be cured via chemical means, thermally, or by exposure to UV or electron beam radiation.
Owner:ASHLAND LICENSING & INTPROP LLC
Radiation-curable inks for flexographic and screen-printing applications from multifunctional acrylate oligomers
InactiveUS7291658B2Excellent gloss and adhesion propertyHigh glossLiquid surface applicatorsMixing methodsAcetoacetatesScreen printing
Owner:ASHLAND LICENSING & INTPROP LLC
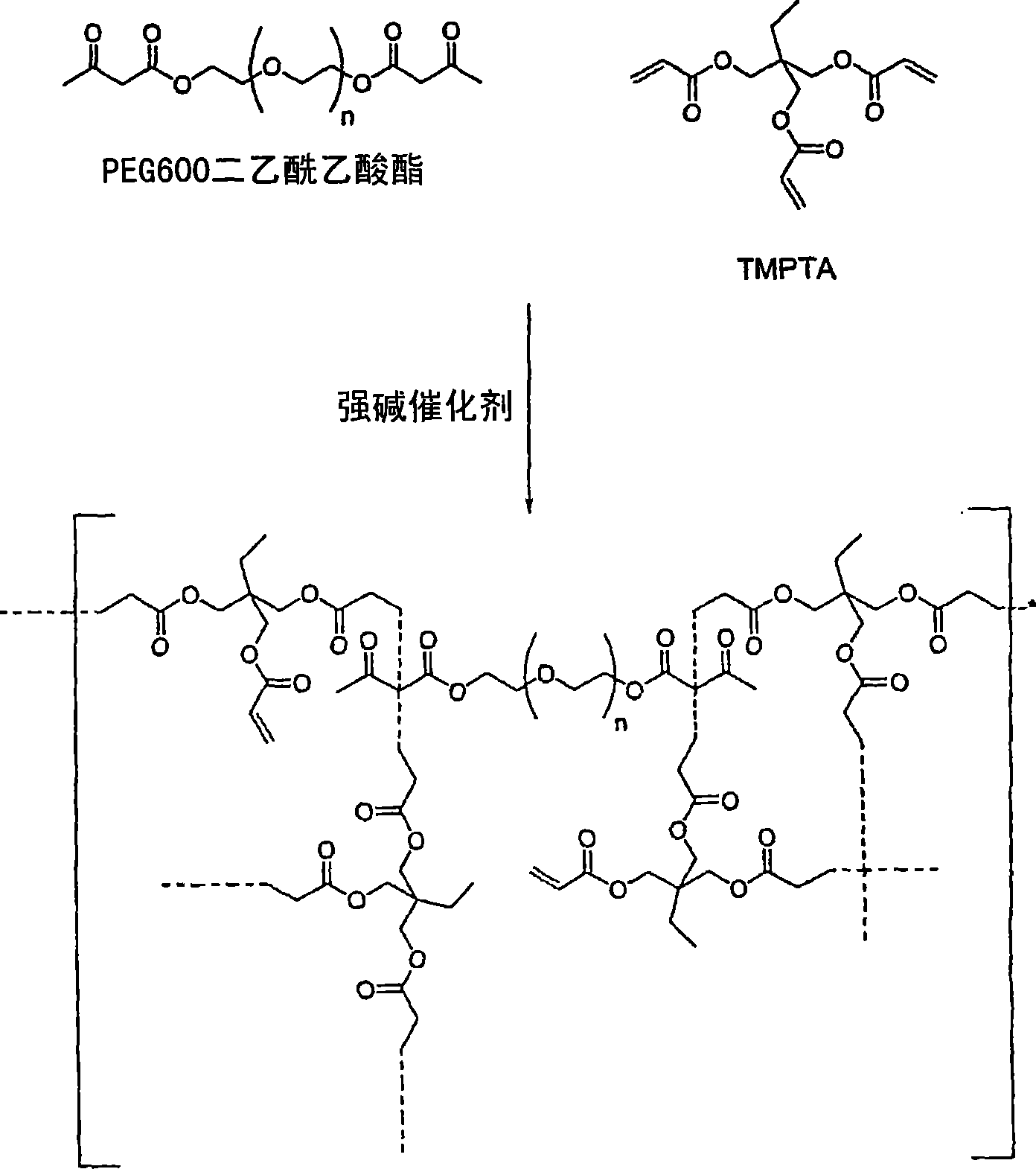
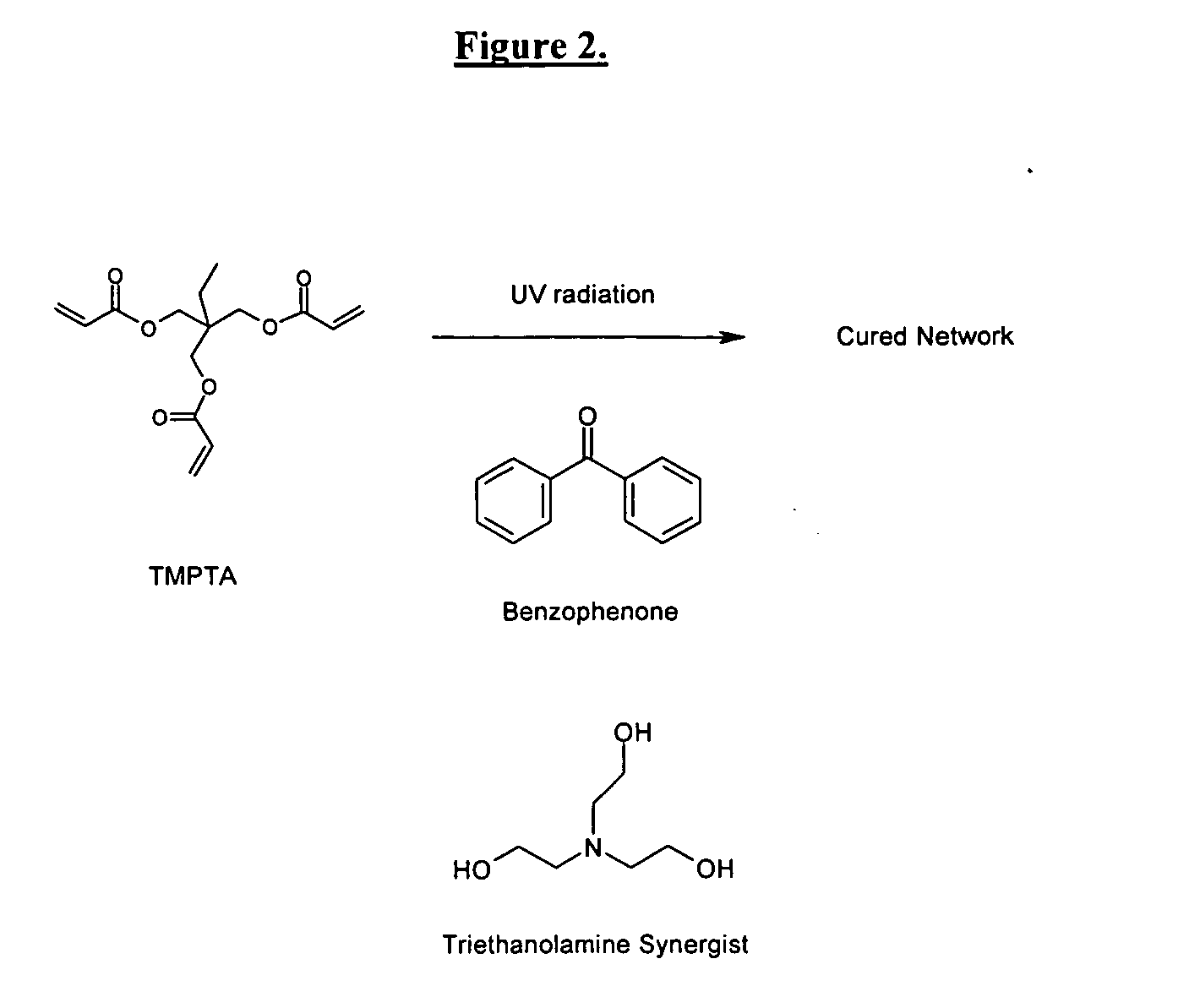
Self-photoinitiating water-dispersible acrylate ionomers and synthetic methods
The invention detailed herein comprises a family of novel multifunctional acrylate ionomeric resins, which are water-dispersible, and have built-in photoinitiator. The inventive resins are made self-photoinitiating by their reaction with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds that can participate in the Michael addition reaction as “Michael donors.” These water-dispersible resins cure under standard ultraviolet (UV) cure conditions to give tack-free coatings without the addition of traditional photoinitiators. The present invention further relates to the use of these resins in coatings.
Owner:ASHLAND LICENSING & INTPROP LLC
New preparation process of 6-chloro-2-hydroxyquinoxaline
InactiveCN102180840AReduce dosageReduce wasteOrganic chemistryHydrazine compoundReaction temperature
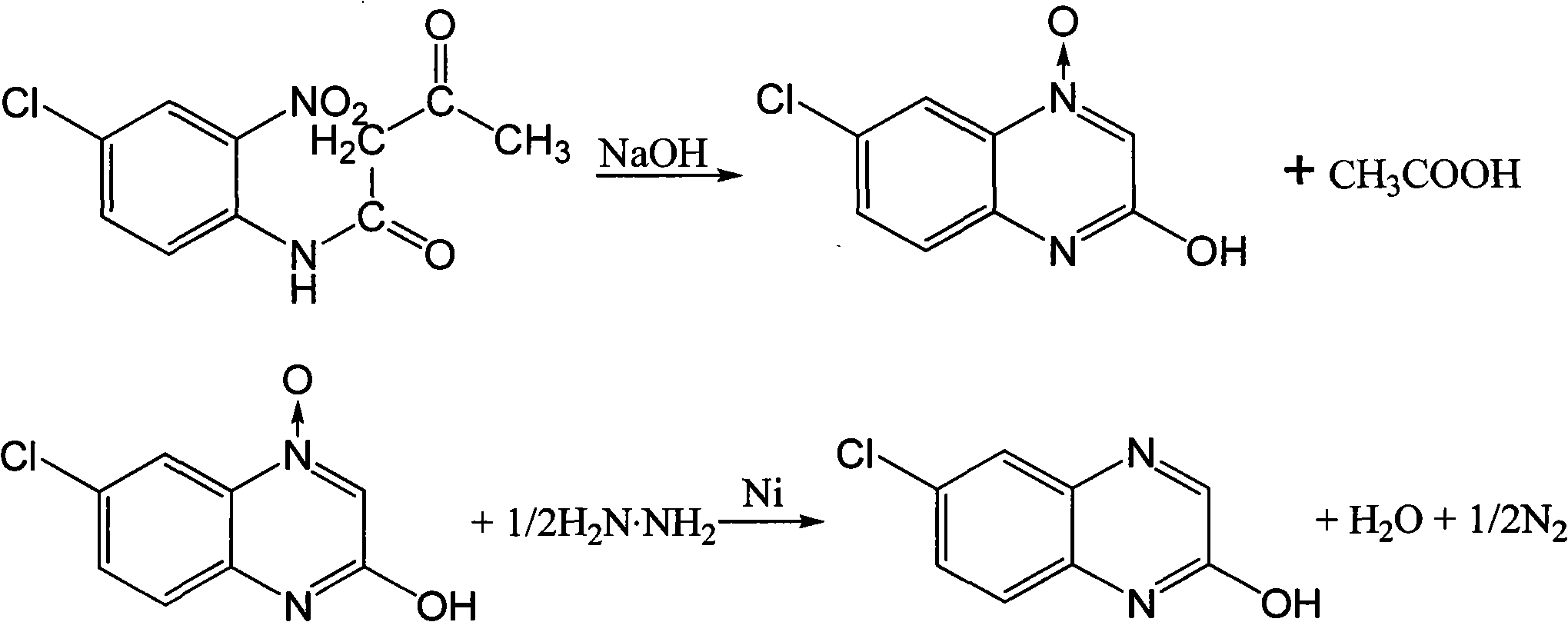
The invention provides a new preparation process of 6-chloro-2-hydroxyquinoxaline. In the process, in a mixed solvent with a catalyst Raney nickel, hydrazine hydrate is utilized to reduce closed-ring substances, which are formed by chloro-o-nitro-acetoacetanilide in an alkali solution, to generate the 6-chloro-2-hydroxyquinoxaline. The invention is characterized in that a one-pot method is adopted, and the adopted mixed solvent is ethanol plus water. The process reduces the consumptions of acid and alkali, simplifies the reactor, lowers the reaction temperature and shortens the reaction time, and the total yield of the product is up to higher than 83%.
Owner:ANHUI FENGLE AGROCHEM
Method for reducing generation of by-product ethyl 3-(phenylamino)but-2-enoate in production of N-acetoacetanilide
ActiveCN104292122ASuppress generationReduce consumptionOrganic compound preparationCarboxylic acid amides preparationChemical industryDistillation
The invention provides a method for reducing generation of a by-product ethyl 3-(phenylamino)but-2-enoate in production of N-acetoacetanilide, and belongs to the field of chemical industry. The method takes ethanol as a solvent, and ethanol can be recycled and reused; in a distillation process of an ethanol mother liquor obtained from filtration, ketene dimer and ethanol are subjected to a reaction to generate ethyl acetoacetate, and phenylamine and ethyl acetoacetate are subjected to a reaction to generate ethyl 3-(phenylamino)but-2-enoate; ethyl 3-(phenylamino)but-2-enoate easily makes N-acetoacetanilide caked, and strong acid and medium-strong acid acidic catalysts are added in the ethanol filtrate obtained from filtration, so that the generation of ethyl 3-(phenylamino)but-2-enoate can be effectively inhibited, a product crystal is optimized, product cracking in a placement process is prevented, the appearance and performance of the product are improved, besides, the product yield and the product purity are increased, and consumption of phenylamine and ketene dimer is reduced.
Owner:NANTONG ACETIC ACID CHEM
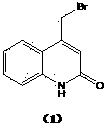
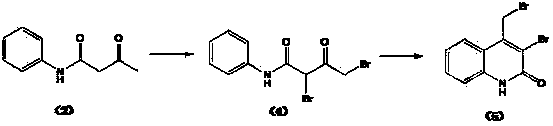
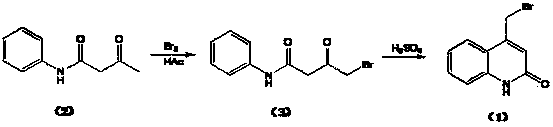
Preparation method of 4-bromomethylquinoline-2(H)-ketone
The invention discloses a preparation method of 4-bromomethylquinoline-2(H)-ketone. The preparation method comprises the following steps of I, with acetoacetanilide as a raw material, dissolving acetoacetanilide into an organic solvent, dropwise adding bromine twice, evaporating the solvent after the reaction is ended, adding water, regulating the pH value to 6-7 by using alkaline, centrifuging, washing to obtain a crude product, pulping the obtained crude product by using alcohol and drying to obtain acetoacetanilide bromide; II, slowly adding acetoacetanilide bromide obtained in the step I into concentrated sulfuric acid, after the reaction is ended, dropwise adding the reaction solution into water under cooling, centrifuging, washing, adding the obtained solid into water, regulating the pH value to 6-7 by using alkaline under cooling, centrifuging, washing to obtain a crude product, pulping the crude product by using alcohol, centrifuging and drying to obtain 4-bromomethylquinoline-2(H)-ketone. By using the preparation method, the problems of too many dibromides generated by bromination reaction and low product purity in the prior art are solved; in addition, the preparation method is high in product yield and product quality, low in production cost and capable of realizing large-scale industrial production.
Owner:SUZHOU TIANMA SPECIALTY CHEM
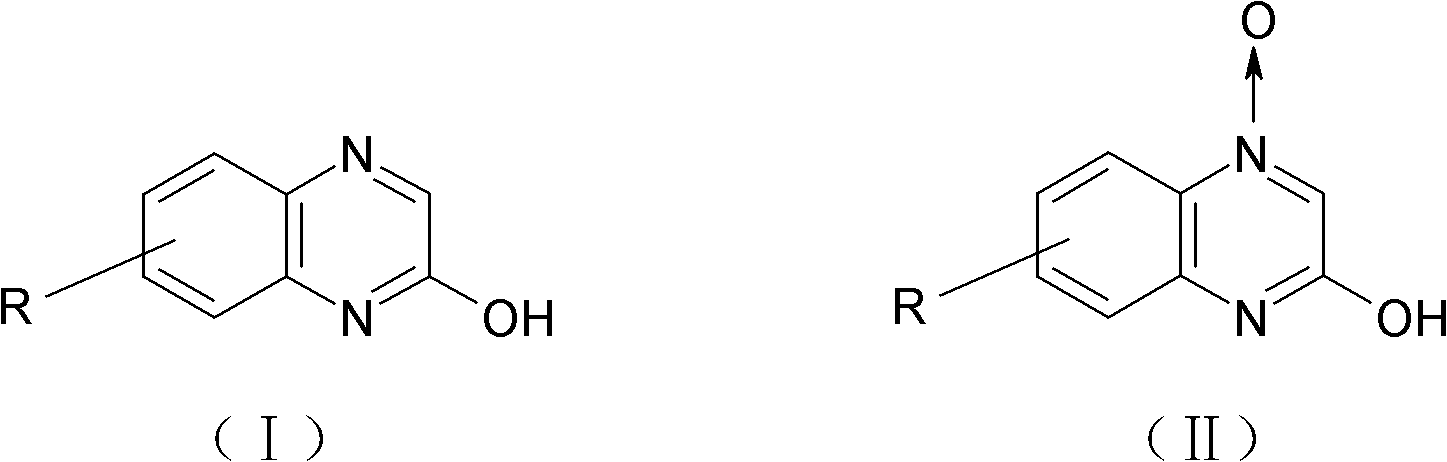
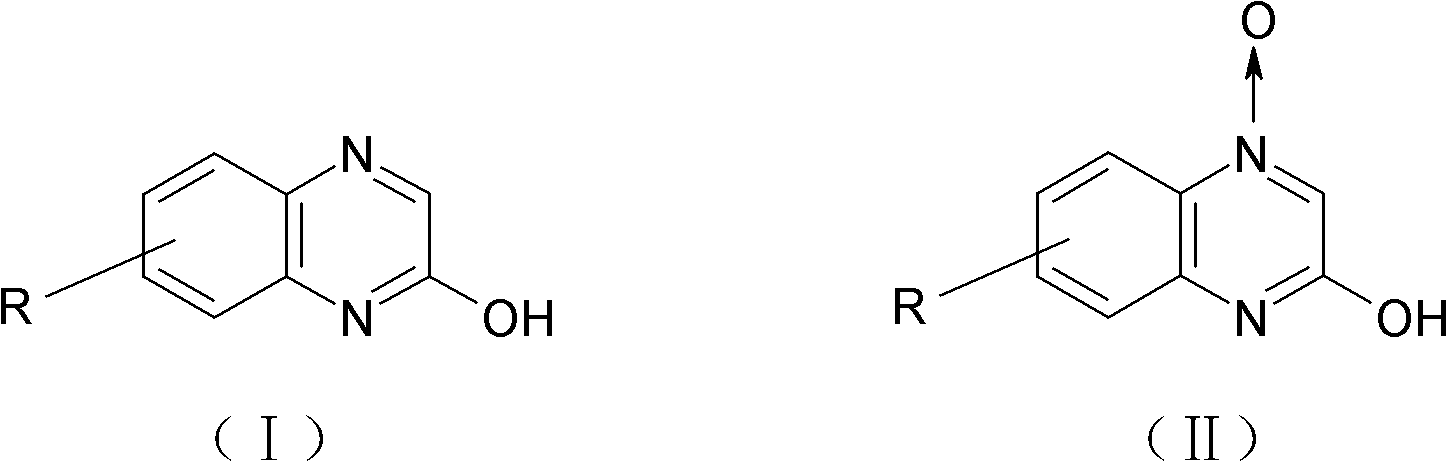
Method for preparing 2-quinoxalinol
The invention discloses a method for preparing 2-quinoxalinol, which comprises the following steps: preparing 2-quinoxalinol-4-oxide by the cyclization of chloro-m-nitroacetoacetanilide serving as a raw material in the presence of a hydroxide; and preparing 2-quinoxalinol by reducing the 2-quinoxalinol-4-oxide with phosphorous acid or trimethyl phosphate serving as a reducer. In the invention, the phosphorous acid or trimethyl phosphate is used as a reducer to reduce 2-quinoxalinol-4-oxide with high selectivity to prepare 2-quinoxalinol, so the byproducts are reduced, and product yield and purity are high; the conditions of the reduction reaction are mild, and the possibility of explosion generated by low-pressure catalytic hydrogenation with hydrogen serving as a reducer is lowered; because the phosphorous acid or trimethyl phosphate commodity serving as the reducer is cheap and readily available, the production cost of the 2-quinoxalinol is reduced; and as the reaction raw materials and the products produced in the reaction process are nontoxic, the method contributes to environmental protection.
Owner:ZHEJIANG HETIAN CHEM CO LTD +1
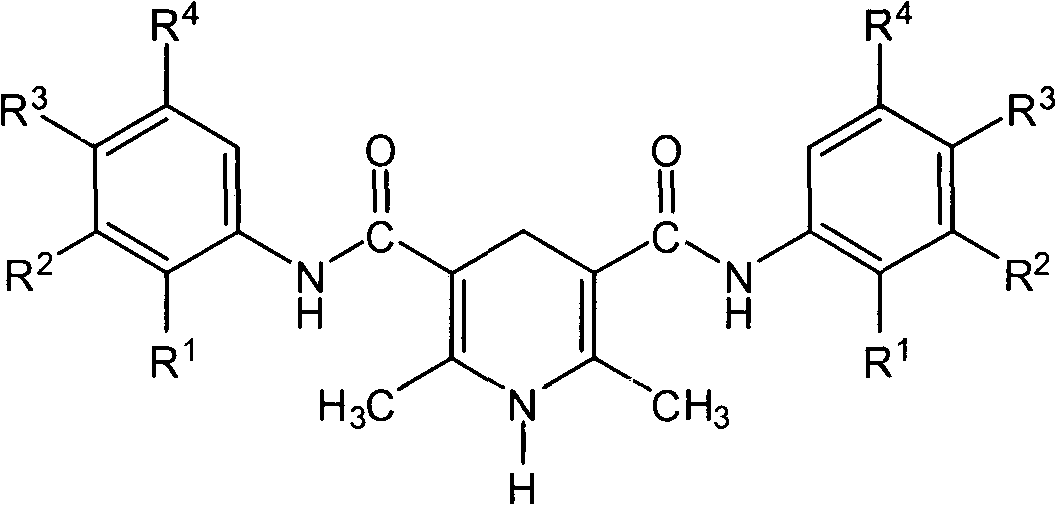
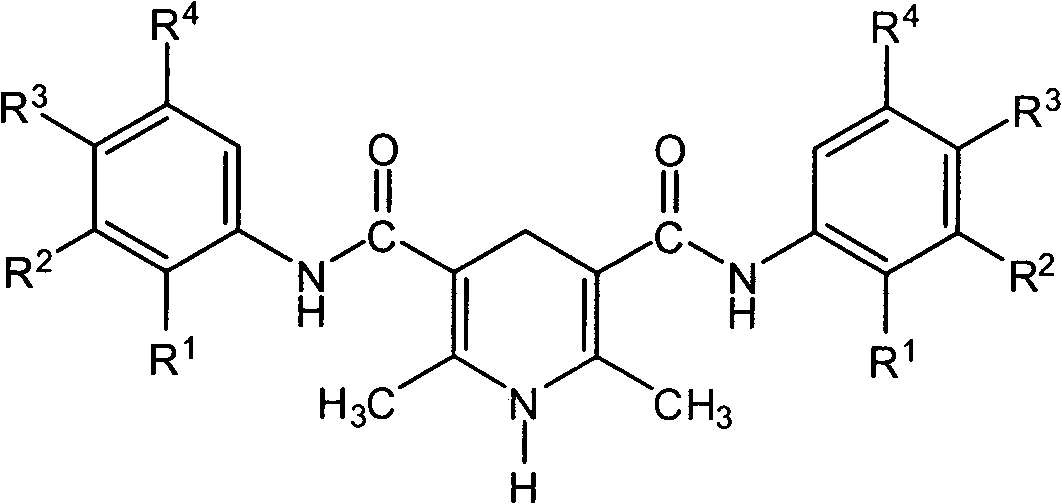
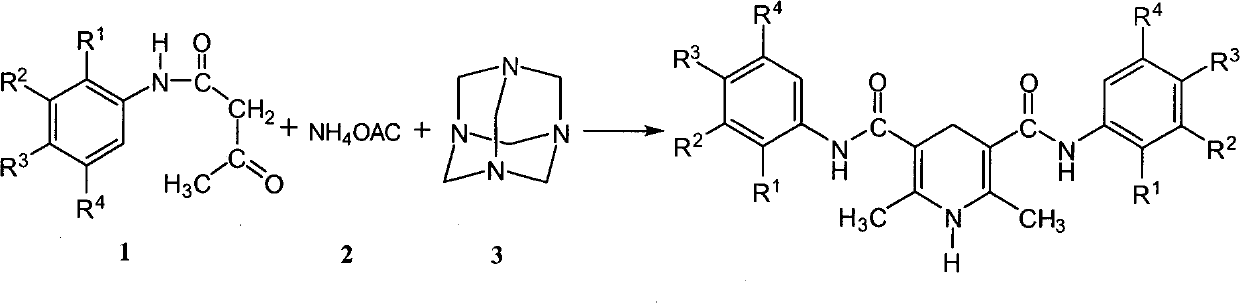
1,4-dihydropyridine compound including acetoacetanilide structure and preparation method thereof
InactiveCN102304078AThe synthesis method is simple and fastEasy to derivatizeOrganic chemistryDihydropyridineAcetoacetanilide
The invention belongs to the field of 1,4-dihydropyridine compounds and in particular relates to a 1,4-dihydropyridine compound including an acetoacetanilide structure and a preparation method thereof. The 1,4-dihydropyridine compound is shown as a structural general formula in the specification. The preparation method comprises the following steps of: taking an acetoacetanilide derivative, ammonium acetate, hexamine or formaldehyde as raw materials, carrying out condensation reaction under a heating condition; and obtaining an objective product after purifying. The method for synthesizing the compound in the invention is simple and rapid. A lot of compound libraries can be synthesized in a short period. The method is applied to researching the molecular diversity of the compound.
Owner:NANJING UNIV OF TECH
Environment-friendly synthetic method of 4-bromomethyl quinolinone
ActiveCN105753781AProduced noEase of mass productionOrganic chemistryOrganic solventRoom temperature
The invention discloses an environment-friendly synthetic method of 4-bromomethyl quinolinone. The environment-friendly synthetic method comprises the following steps: adding acetoacetanilide bromide as a raw material into an anhydrous organic solvent; taking out moisture generated in reaction by virtue of the organic solvent as a water carrier while heating, condensing the moisture-containing organic solvent, adding the organic solvent into a reaction kettle filled with phosphorus pentoxide, and carrying out dehydration under stirring; continuously distilling the organic solvent in the reaction kettle filled with phosphorus pentoxide, returning the distilled anhydrous organic solvent to an acetoacetanilide bromide reaction kettle as the water carrier, carrying out dehydration cyclization on acetoacetanilide bromide so as to generate 4-bromomethyl quinolinone, cooling the system to the room temperature, carrying out filter pressing by directly utilizing a filter press, washing by virtue of an organic solvent, and drying, so as to obtain the product. According to the environment-friendly synthetic method, the yield of reaches 95% or above, and the purity of 4-bromomethyl quinolinone is more than or equal to 99.5%; and the product quality is good, waste acid and wastewater are not generated, the production efficiency is high, the cost is low, and large-scale production is easily realized.
Owner:陈科
Preparation method of crystalline N-acetoacetanilide
InactiveCN107652199AEliminate the phenomenon of cloggingUniform crystal shapeOrganic compound preparationCarboxylic acid amide separation/purificationSolventAniline
The invention discloses a preparation method of crystalline N-acetoacetanilide. The method comprises the following steps: reacting aniline with diketene, carrying out condensation on the aniline and diketene, adding an acid accounting for 0.01-0.1% of the weight of the above obtained condensation solution into a condensation kettle, heating the obtained solution to 40-70 DEG C, starting the vacuumof a system, gradually increasing the vacuum degree to 610-460 mmHg from 760 mmHg, keeping the vacuum degree at 610-460 mmHg for 10-50 min, and continuously increasing the vacuum degree to 385-310 mmHg; and decreasing the temperature to 40 DEG C or less, continuously increasing the vacuum degree to 160-85 mmHg within 0.5-2 h, finally reducing the vacuum degree to 10-5 mmHg, keeping the vacuum degree for 15-30 min, shutting down a vacuum pump, and carrying out system emptying, centrifuging and drying to obtain the finished uniformly-crystalline quicksand-like N-acetoacetanilide product. A specific crystallization technology adopting a crystallization assistant is developed in the invention, so the product has the advantages of easiness in crystallization formation, high purity, uniform particles, good fluidity, and no agglomeration after long-term storage. The quality of downstream products is improved.
Owner:NANTONG ACETIC ACID CHEM
Multifunctional acrylate oligomers as pigment grinding vehicles for radiation-curable ink applications
Owner:ASHLAND LICENSING & INTPROP LLC
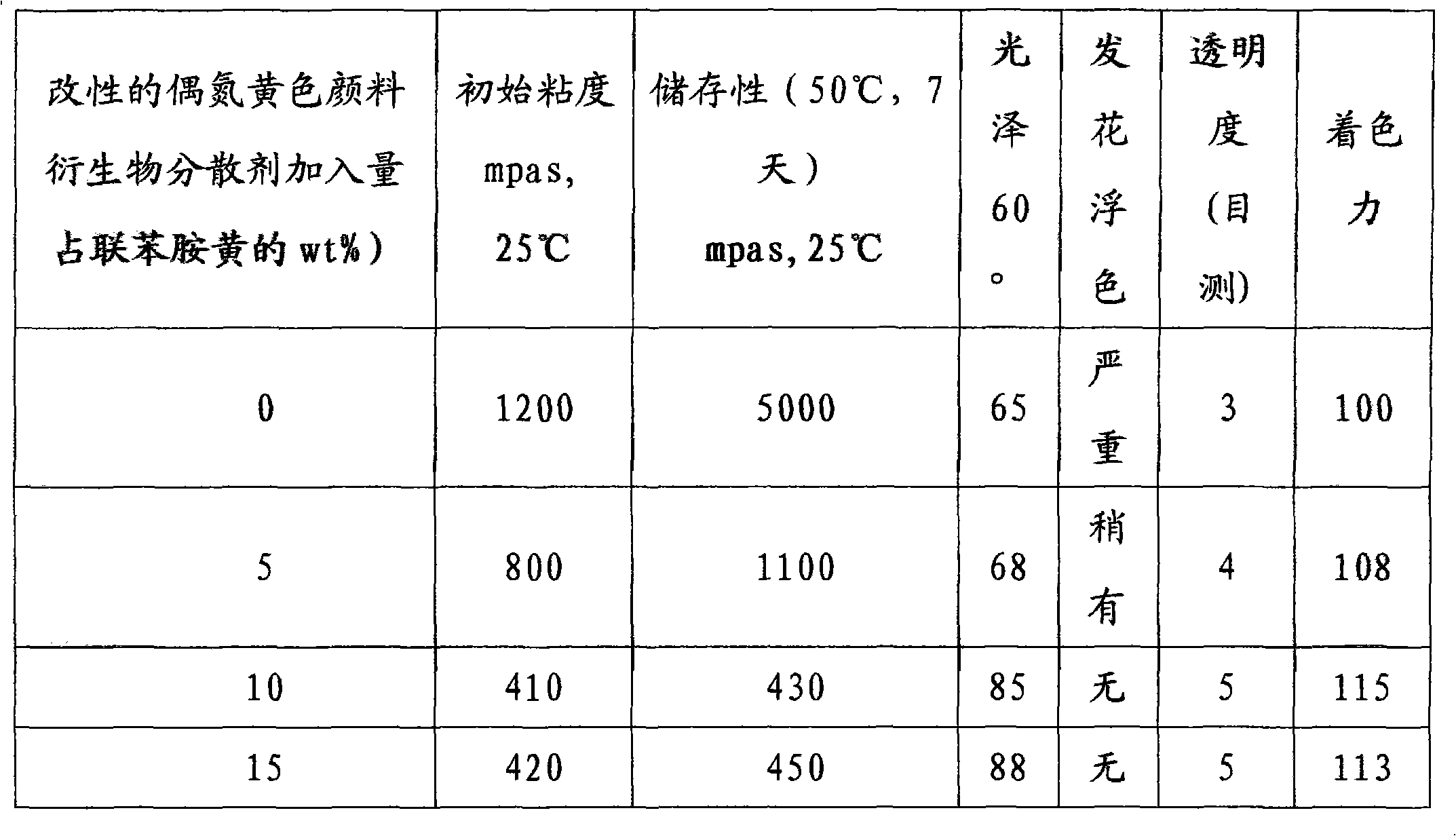
Preparation method of modified azo yellow pigment derivative dispersant as well as prepared derivative dispersant and application thereof
The invention discloses a preparation method of a modified azo yellow pigment derivative dispersant as well as a prepared derivative dispersant and application thereof. The preparation method comprises the following steps of: (1) dissolving 4-aminobenzene phosphonic acid into water, adding alkali and ketene dimer, and stirring, reacting and drying to obtain 4-acetoacetyl aminobenzene phosphonate; (2) adding 3,3-dichlorobenzidine into a hydrochloric acid solution, stirring, adding a nitrite solution and reacting to obtain a diazonium solution; (3) under a stirring state, adding acetoacetanilide and the 4-acetoacetyl aminobenzene phosphonate into an alkali solution, and dropping an acetic acid solution to obtain a coupling solution; (4) adding the diazonium solution into the coupling solution and coupling to obtain azo yellow pigment derivative color paste; and (5) adding fatty amine into the azo yellow pigment derivative color paste, and stirring, filtering, washing by water and drying to obtain a modified azo yellow pigment derivative dispersant product. When the dispersant is used, the added quantity of the dispersant occupies 5-15 weight percent of the quantity of azo yellow pigment.
Owner:WINBOS NEW MATERIALS
Efficient and environmentally-friendly method for preparing 6-chloro-2-quinoxaline phenol
The invention discloses an efficient and environmentally-friendly method for preparing 6-chloro-2-quinoxaline phenol. The method is characterized by comprising the following steps of: (1) dissolving p-chloro-o-nitroacetoanilide into a sodium hydrate or potassium hydrate solution, and reacting at the temperature of 70-90 DEG C for 2-8 hours; (2) adding an appropriate amount of catalyst, wherein the catalyst is ferric hydrate, FeOH / C or Fe(OH)3 / C; and (3) dropwise adding a hydrazine hydrate serving as a reducing agent, controlling the dripping temperature at 70-90 DEG C, preserving heat for 8 hours, cooling after reacting, filtering, and drying to obtain 6-chloro-2-quinoxaline phenol serving as a finished product. The method is characterized in that: a cheap and safe iron / active carbon catalyst is used, the hydrazine hydrate is taken as the reducing agent, nitrogen gas which is harmless to the environment is discharged in a reaction, an entire reaction system is efficient and environmentally-friendly and has low cost, and the method is convenient for industrial popularization.
Owner:JIANGSU FENGSHAN GROUP
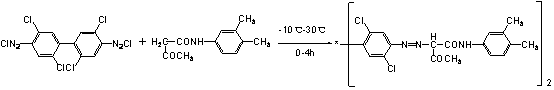
Preparation process of benzidine yellow
The invention discloses a preparation process of benzidine yellow, which is characterized by comprising the following steps of: adding 1150-1250L of water, 95-110 KG of acetoacetanilide and 2.5-3.5 KG of an assistant into a coupling barrel, mixing and beating the obtained mixture for 60-70 min, adding 180-200 KG of 56-100% sodium acetate and 15-30% filler into the coupling barrel, and adding ice blocks into the coupling barrel so as to cool the obtained object to 15-17 DEG C; adding 10-13 KG of a hydrochloric acid with the concentration of 30-33%, and adjusting the pH of the obtained mixture to be 6-7; adding 60-80 KG of 100% diazo fluid into the coupling barrel, controlling the temperature at 7-12 DEG C, and controlling the pH at 3.5-4.0; in the later period of adding the diazo liquid, carrying out detection by using a ring lubricating test method; and after the reaction is completed, stopping adding the diazo liquid, heating the liquid material to 90-120 DEG C by using steam, and carrying out heat preservation for 25-35 min. A pigment prepared according to the preparation process of the benzidine yellow is uniform in color strength and more stable in crystal form.
Owner:瑞安宝源化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com