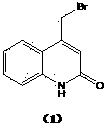
Preparation method of 4-bromomethylquinoline-2(H)-ketone
A technology of bromomethylquinoline and liquid bromine, applied in the direction of organic chemistry and the like, can solve problems such as excessive dibromide, achieve the effects of improving purity, optimizing bromination reaction, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
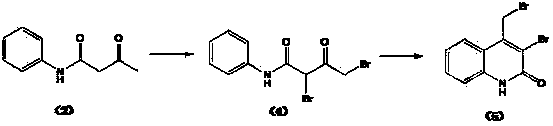
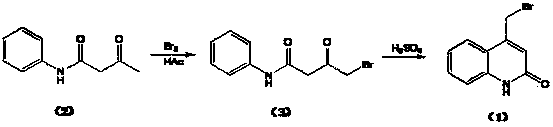
[0031] Preparation of brominated acetoacetanilide shown in formula (3): at room temperature, dissolve 270kg of acetoacetanilide shown in formula (2) in 1200kg of dichloroethane, add 170kg of liquid bromine dropwise at 20~30°C, After 2 hours of dripping, keep stirring at 50~55°C for 2 hours, cool down to 20~30°C, add the remaining 86kg of liquid bromine dropwise, finish dropping in 2 hours, keep stirring at 50~55°C for 2 hours. After the reaction is completed, concentrate to dryness under reduced pressure, add 400kg of water, and at 10-20°C, add dropwise 20% sodium hydroxide solution to adjust the pH to 6-7, centrifuge, and wash with water until the pH is 7. Add the crude product to 1200kg of methanol, beat at 50-55°C for 1 hour, cool to 0°C, centrifuge, wash with cold methanol, and obtain 319kg of white solid with a yield of 81.7% and a purity of 97.6%. The dibromide represented by formula (4) 1.4% (HPLC area normalization method). Elemental analysis: found (calculated), C 45...
Embodiment 2
[0033] The preparation of 4-bromomethylquinolin-2(H)-one shown in formula (1): Slowly add brominated acetoacetanilide shown in 312kg formula (3) to 2400kg concentrated sulfuric acid, control temperature 10~ 20°C, after the addition, keep the reaction at 15~20°C for 3 hours. After the reaction is over, add the feed liquid to 2400kg of ice water under cooling, control the temperature below 20°C, stir for 30 minutes, centrifuge, wash with water to pH 2~3, add the solid to 1500kg of water, add 20% hydrogen dropwise at 10~20°C Adjust the pH to 6~7 with sodium oxide solution, centrifuge, wash with water until the pH is 7, and obtain the crude product. Add 2400kg of methanol to the crude product, beat at 20-30°C for 1 hour, centrifuge, wash with methanol, and dry under vacuum at 50°C to obtain 269.7kg of white powdery solid with a yield of 93.0% and a purity of 99.6%. -Bromo-4-bromomethylquinolin-2(H)-one 0.13% (HPLC area normalization method). Elemental analysis: found (calculated...
Embodiment 3
[0035]Preparation of brominated acetoacetanilide shown in formula (3): at room temperature, dissolve 45g of acetoacetanilide shown in formula (2) in 300g of chloroform, add 30g of liquid bromine dropwise at 20~30°C, drop for 2 hours After completion, keep stirring at 50-55°C for 2 hours, cool down to 20-30°C, add the remaining 16g of liquid bromine dropwise, finish dropping in 2 hours, and keep stirring at 50-55°C for 2 hours. After the reaction is completed, concentrate to dryness under reduced pressure, add 100 g of water, and at 10-20°C, add dropwise 10% sodium carbonate solution to adjust the pH to 6-7, centrifuge, and wash with water until the pH is 7. Add the crude product to 360g of isopropanol, beat at 50-55°C for 1 hour, cool to 0°C, centrifuge, and wash with cold isopropanol to obtain 50.2g of white solid with a yield of 77.2% and a purity of 96.5%. 2.7% of the indicated dibromide (HPLC area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com