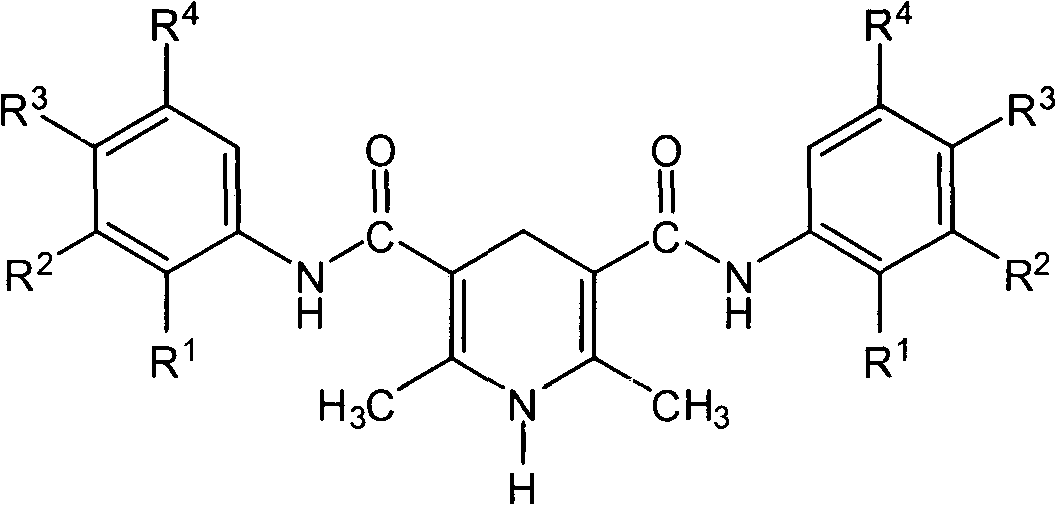
1,4-dihydropyridine compound including acetoacetanilide structure and preparation method thereof
A technology of acetoacetanilide and dihydropyridine, applied in the field 1, can solve problems such as difficult derivatization, unfavorable molecular diversity research, cumbersome synthesis methods, etc., and achieve good drug dynamics and kinetic properties, good drug use prospects, The effect of the simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 2,6-dimethyl-3,5-[N,N-diphenyl]dicarboxamido-1,4-dihydropyridine
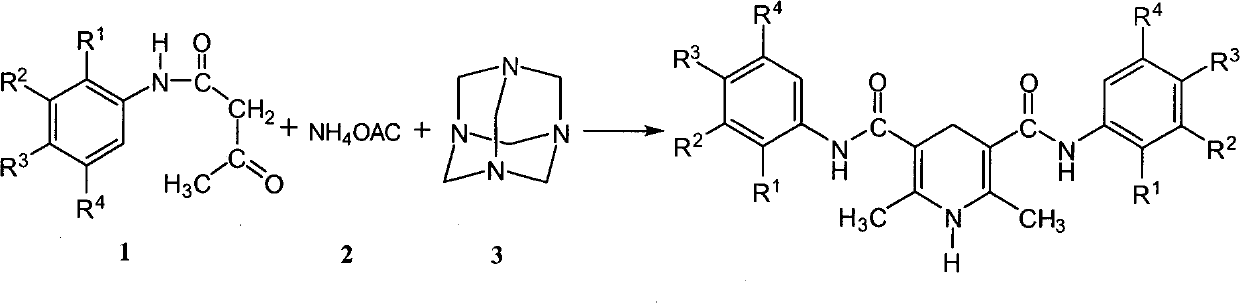
[0029] Dissolve 1.77g (0.01mol) of 2,4-dimethylacetoacetanilide in 40mL of ethanol, add 1.40g (0.01mol) of hexamethylenetetramine and 1.54g (0.02mol) of ammonium acetate to the solution 10mL aqueous solution, heated at 55-70°C for 1.5h. Most of the solvent was removed by rotary evaporation, and a large amount of light yellow solid was precipitated, which was cooled to room temperature and then suction filtered. The precipitate was first washed 3 times with 2 mL of 50% (V / V) methanol-water solution, then washed 3 times with 5 mL of water, and dried to obtain 1.53 g of light yellow solid powder with a yield of 83.3%. The melting point of the product is 224-226°C. The purity of the product analyzed by HPLC normalization method was 99.5%.
[0030] The structural analysis is as follows:
[0031] 1 H-NMR (500MHz, DMSO-d 6 )δ: 2.02(s, 6H, 2×CH 3 ), 3.41 (s, 2H, CH 2 ), 6.98~7.64 (m, 10H, 2×...
Embodiment 2
[0034] The synthesis of 2,6-dimethyl-3,5-[N,N-bis(2,5-dimethoxy-4-chloro)phenyl]dicarboxamido-1,4-dihydropyridine will 2.72g (0.01mol) of 2,5-dimethoxy-4-chloroacetoacetanilide was dissolved in 40mL of isopropanol, and 1.44mL (0.02mol) of 37-40% formaldehyde solution and 5mL of dissolved 1.54g (0.02mol) ammonium acetate aqueous solution was heated at 55-70°C for 2h. Most of the solvent was evaporated by rotary evaporation, and a large amount of yellow solid precipitated out, which was cooled to room temperature and suction filtered. The precipitate was washed 3 times with 2 mL of 50% (V / V) methanol-water solution and then 3 times with 5 mL of water. After drying, 1.96 g of light yellow solid powder was obtained with a yield of 73.3%. The melting point of the product is 239-241°C. The purity of the product analyzed by HPLC normalization method was 98.5%.
[0035] The structural analysis is as follows:
[0036] 1 H-NMR (500MHz, CDCl 3 )δ: 2.43(s, 6H, 2×CH 3 ), 3.65(s, 6H,...
Embodiment 3
[0039] Synthesis of 2,6-Dimethyl-3,5-[N,N-bis(potassium p-sulfonate)phenyl]dicarboxamido-1,4-dihydropyridine
[0040] Dissolve 2.95g (0.01mol) of potassium p-sulfonate acetoacetanilide in 40mL of 50% (V / V) methanol-water, add 2.80g (0.02mol) of hexamethylenetetramine and 3.08g of (0.04mol) 10mL aqueous solution of ammonium acetate, heated at 55-70°C for 2.5h. Most of the solvent was removed by rotary evaporation, and a large amount of light yellow solid was precipitated, which was cooled to room temperature and then suction filtered. The precipitate was first washed 5 times with 2 mL of 50% (V / V) methanol-water solution, and dried to obtain 2.43 g of light yellow solid powder with a yield of 83.4%. The purity of the product analyzed by HPLC normalization method was 98.7%.
[0041] The structural analysis is as follows:
[0042] 1 H-NMR (500MHz, DMSO-d 6 )δ: 2.03(s, 6H, 2×CH 3 ), 3.42 (s, 2H, CH 2 ), 7.48~7.61 (m, 8H, 2×PhH), 7.84 (s, H, NH), 9.13 (s, 2H, 2×NH).
[0043] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com