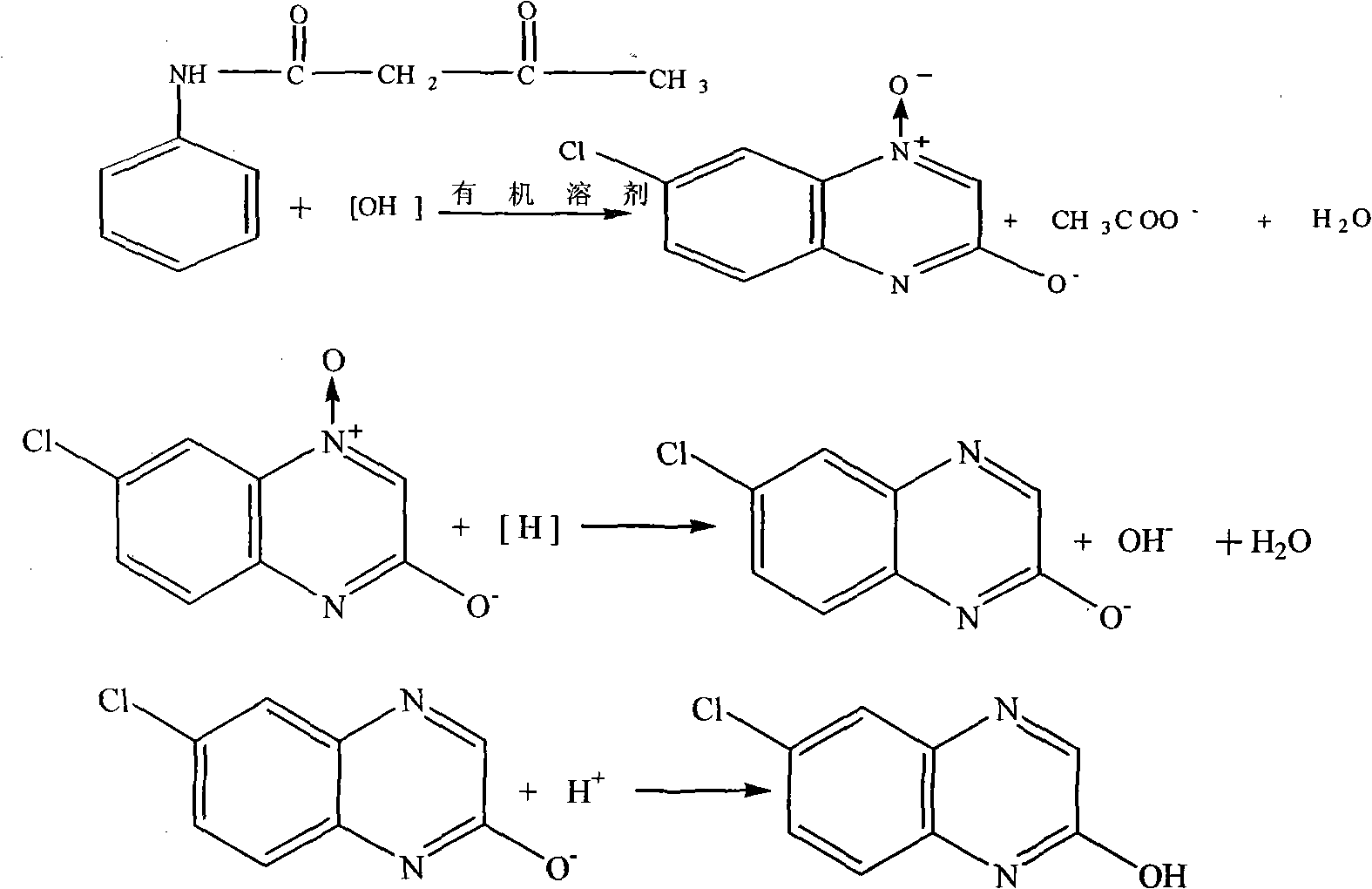
Synthesis method of 2-chloro-6-chloroquinoxaline
A technology of hydroxyquinoxaline and synthetic method, which is applied in the field of organic synthesis, can solve problems such as inability to obtain social and economic benefits, unfavorable large-scale industrial production of quizalofop-pyl-ethyl, and achieve saving of investment and production costs and reduction of alkaline wastewater , Stable effect of production and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Closed loop reaction stage:
[0025] Put 300kg of solid sodium hydroxide into a 5000L reactor, stir 1000kg of methanol solution, prepare an alkaline solvent, pass cooling water to cool down to 25±5°C, and then put in 400kg of p-chloro-o-nitroacetoacetanilide, at 25±5 React at ℃ for 3 hours, then slowly raise the temperature to 60-65℃, react for 2 hours, take a tracking sample, and use liquid chromatography to analyze whether the reaction of p-chloro-o-nitroacetylacetanilide is complete. If the tracking result is p-chloro-o-nitroacetyl If the content of acetanilide is less than 0.1%, it can be processed. If the tracking result shows that the content of p-chloro-o-nitroacetoacetanilide is > 0.1%, the reaction time needs to be extended for 2 hours, and then follow up until the reactant is p-chloro-o-nitroacetoacetanilide Aniline < 0.1% can be processed in the next step;
[0026] Reduction reaction stage:
[0027] Put 200kg of sodium hydrosulfide and 500kg of methanol int...
Embodiment 2
[0031] Closed loop reaction stage:
[0032] Put 180kg of potassium hydroxide solid and 800kg of acetone solution into a 3000L reaction kettle, start stirring, prepare an alkaline solvent, pass cooling water to cool down to 25±5°C, and then put in 260kg of p-chloro-o-nitroacetoacetanilide, at 25±5°C React at 5°C for 3 hours, then slowly raise the temperature to 60-65°C, react for 2 hours, take a tracking sample, and use liquid chromatography to analyze whether the reaction of p-chloro-o-nitroacetoacetanilide is complete, if the tracking result is p-chloro-o-nitro If the content of acetoacetanilide is less than 0.1%, it can be processed. If the tracking result shows that the content of p-chloro-o-nitroacetoacetanilide is more than 0.1%, it is necessary to prolong the reaction time before tracking. Until the reactant p-chloro-o-nitroacetoacetanilide content reaches the requirements.
[0033] Reduction reaction stage:
[0034]Put 200kg of sodium hydrosulfide and 500kg of acetone...
Embodiment 3
[0042] Preparation of lye for ring closure reaction:
[0043] Measure 800Kg of methyl tert-butyl ether in a 3000L reactor, turn on the agitator, put in 150Kg of sodium hydroxide, pass cooling water to cool down to 20-30°C, and then put in 400Kg of p-chloro-o-nitroacetoacetanilide, at 20- 30°C heat preservation reaction for 3 hours, slowly raise the temperature to 50°C with steam, and heat preservation reaction for 2 hours;
[0044] Reduction reaction:
[0045] Put 500Kg of methyl tert-butyl ether and 150Kg of potassium borohydride into the reaction kettle, slowly raise the temperature to 60°C with steam, and react under slight reflux for 20 hours. metal salt;
[0046] Acidification reaction stage:
[0047] In the acidification reaction stage, the existing acidification method is used for acidification, and the product content measured by liquid chromatography is 97.1%, and the yield is 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com