New preparation process of 6-chloro-2-hydroxyquinoxaline
A hydroxyquinoxaline, a new process technology, applied in the field of pesticides, can solve the problems of high reaction temperature, long reaction time, complicated dropping device, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
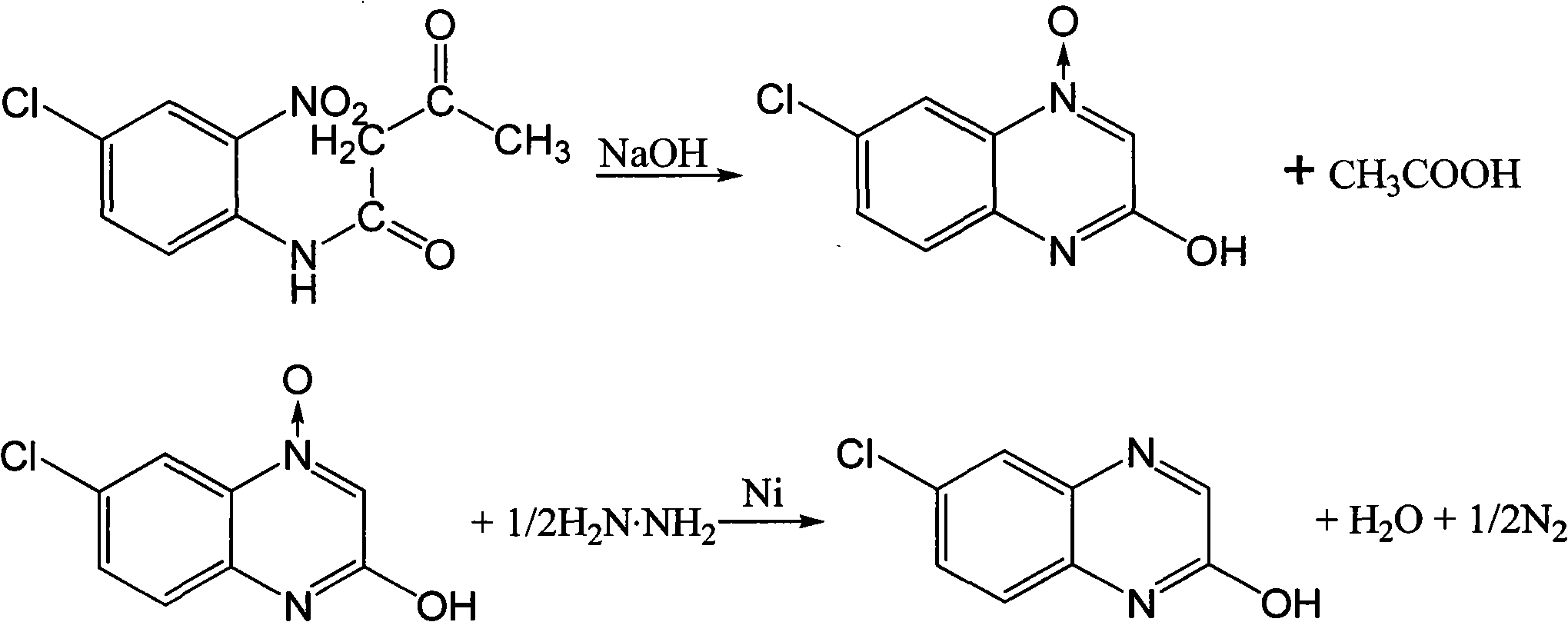
[0006] Embodiment one: a kind of new preparation technology of 6-chloro-2-hydroxyquinoxaline, this technique uses Raney nickel as catalyst, hydrazine hydrate prepares 6-chloro-2-hydroxyquinoxaline as reducing agent, reaction formula is as follows :
[0007]
[0008] In a 500 ml four-necked flask equipped with a thermometer, a stirrer and a condenser tube, add 0.1 mol of p-chloro-o-nitroacetoacetanilide, add 200 ml of 10% sodium hydroxide solution, stir evenly and heat up to 65°C, keep warm After stirring for 1 hour, about 50 ml of ethanol was added, and the temperature of the reaction mixture solution was cooled to 30° C., 0.5 g of catalyst Raney nickel and 5 g of 80% hydrazine hydrate were added, and the mixture was kept stirring for 2 hours. After the reaction is over, filter (the filtered catalyst is used directly next time), distill ethanol from the filtrate under reduced pressure (the evaporated solvent is used directly next time), add an appropriate amount of acid to ...
Embodiment 2
[0009] Embodiment two: in the 500 milliliter four-neck flask that thermometer, stirrer and condenser tube are housed, add the p-chloro-o-nitroacetoacetanilide of 0.1mol, add 200 milliliters of 10% sodium hydroxide solution, stir and heat up to 65 DEG C, after insulated and stirred for 1 hour, add about 50 milliliters of ethanol (recovery in embodiment one), the reaction mixture solution is cooled to 30 DEG C, add catalyst Raney nickel (recovery in embodiment one) 0.5 grams and 5 grams of 80% hydrazine hydrate, Keep warm and stir for 2 hours. After the reaction is over, filter (the filtered catalyst is used directly next time), distill ethanol from the filtrate under reduced pressure (the evaporated solvent is used directly next time), add an appropriate amount of acid to acidify to neutrality, filter, and filter cake Washed with water and dried to obtain 15.0 g of the product, the yield was 83.3% based on p-chloro-o-nitroacetoacetanilide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com