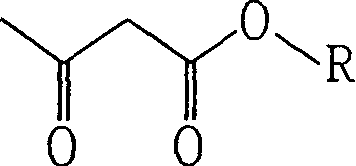
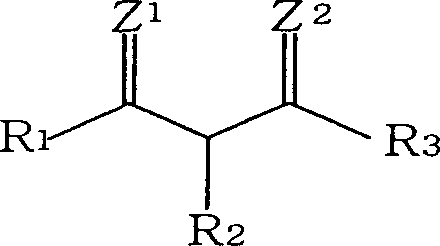
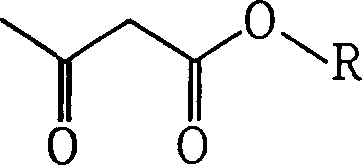
Acetacetic acid alkyl ester metal chelate coating drier and production thereof
A technology of alkyl acetoacetate and metal chelate, which is applied in the direction of drier, chemical instrument and method, coating, etc., can solve the problems of unconducted research and corresponding experiments, and achieve simple preparation process and source Broad, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A: Preparation of n-butyl acetoacetate
[0034] 1. Reaction equation:
[0035]
[0036] 2. Formulation:
[0037] Reactant Mole Mass (g)
[0038] n-Butanol 1.05 77.7
[0039] Diketene 1.0 84
[0040] 98% concentrated sulfuric acid (catalyst) 0.5
[0041] 3. Operation process:
[0042] Put the four-necked flask on the electric constant speed stirrer, install the reflux water separator at the left port, install the constant pressure dropping funnel at the right port, connect the nitrogen inlet pipe at the top port of the dropping funnel, install the thermometer at the fourth port, and react The flask was heated with an electric heating mantle without an open flame.
[0043] Put n-butanol and concentrated sulfuric acid into the reaction bottle according to the recipe, heat up under stirring, and protect with nitrogen gas. Heat to 90°C, and slowly add diketene dropwise after the temperature stabilizes (the reaction exotherms violently, so the rate of addition is co...
Embodiment 2
[0096] A: Preparation of n-pentyl acetoacetate
[0097] 1. Reaction equation:
[0098]
[0099] 2. Formulation:
[0100] Reactant Mole Mass (g)
[0101] n-Pentanol 1.0 88.2
[0102] Diketene 1.0 84.0
[0103] p-toluenesulfonic acid (catalyst) 0.5
[0104] 3. Operation process:
[0105] According to the reaction device of Example 1, drop n-amyl alcohol and p-toluenesulfonic acid into the reaction bottle, heat up under stirring, and protect with nitrogen. Heating to 90°C, and slowly adding diketene dropwise after stabilization (the reaction exothermic is violent, so the rate of addition is controlled so that the temperature is controlled at about 90°C). After diketene is added dropwise, react at 90°C---100°C for 2 hours.
[0106] After the reaction was completed, the reactant was cooled to 50° C., and neutralized to neutrality with 10% aqueous sodium carbonate. Stand to separate layers, remove the water layer, and dry the oil layer with anhydrous magnesium sulfate ove...
Embodiment 3
[0143] A: Preparation of n-hexyl acetoacetate:
[0144] 1. Reaction equation:
[0145]
[0146] 2. Formulation:
[0147] Reactant Mole Mass (g)
[0148] n-Hexanol 1.0 102
[0149] Diketene 1.0 84.0
[0150] Triethylamine (catalyst) 1.5ml
[0151] 3. Operation process:
[0152] According to the reaction device of Example 1, drop into n-hexanol and triethylamine in the reaction flask, heat up under stirring, and protect with nitrogen. Heating to 60°C, and slowly adding diketene dropwise after stabilization (the reaction exothermic is violent, so the rate of addition is controlled so that the temperature is controlled at about 60°C). After the diketene is added dropwise, react at about 60°C for 1 hour, depending on the completeness of the reaction of diketene, and the end point of the reaction is controlled by gas chromatography.
[0153] After the reaction was complete, the reactant was cooled. Distill under reduced pressure to collect the target product fraction at 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com