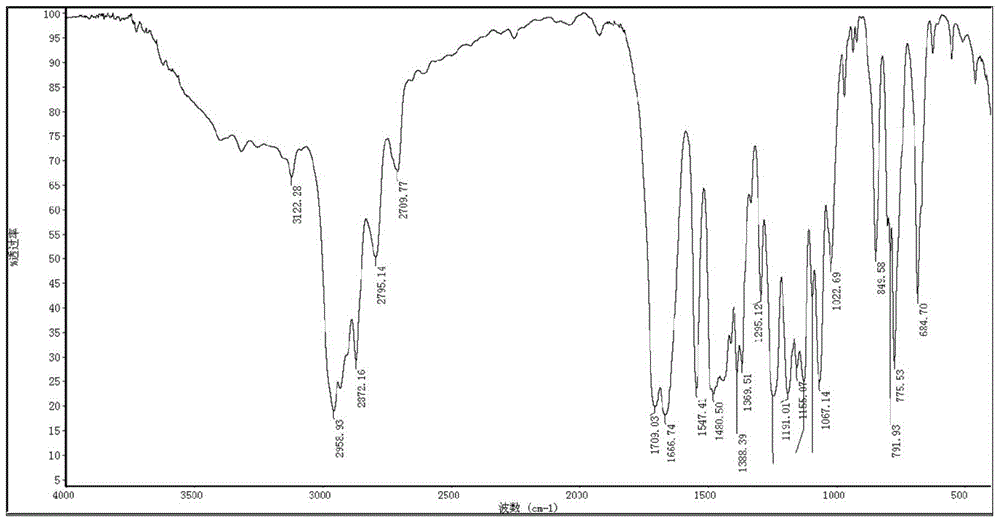
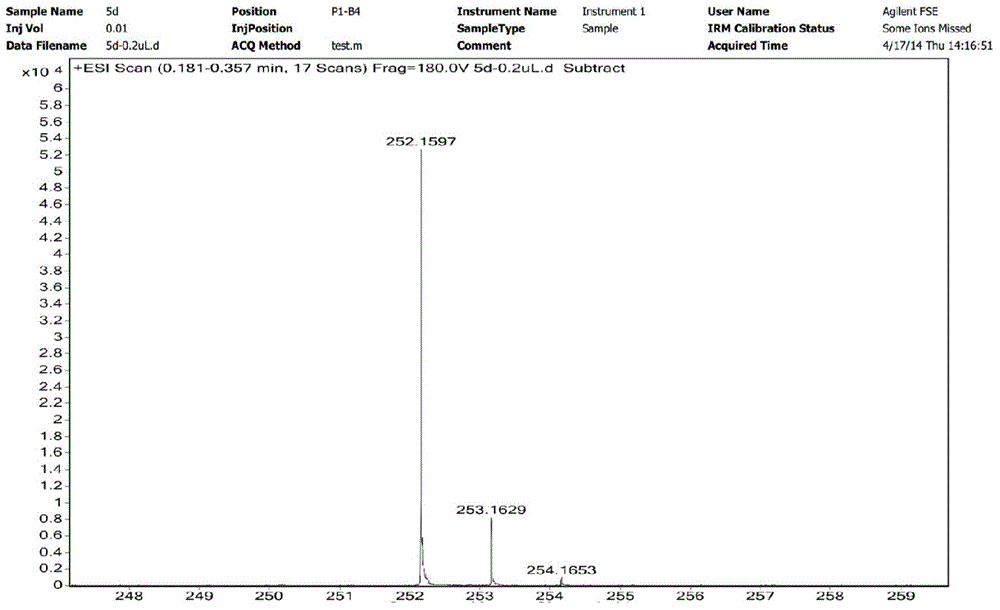
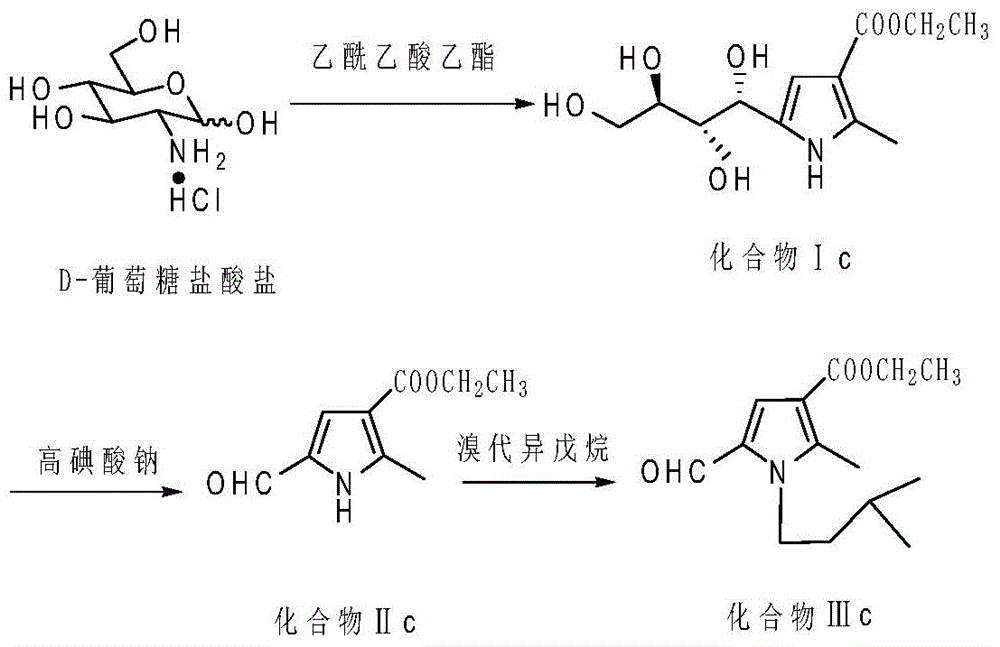
Preparation method for N-alkyl-2-methyl-5-formyl-3-pyrrole formate and application of N-alkyl2-methyl-5-formyl-3-pyrrole formate in cigarette flavoring
A technology of pyrrole formate and formyl, which is used in the application of cigarette flavoring, and the field of preparation of N-hydrocarbyl-2-methyl-5-formyl-3-pyrrole formate, which can solve the problem of cigarette product easy Loss of characteristics, volatilization of aroma substances, loss of aroma and other problems, to achieve the effect of plump smoke, comfortable aftertaste, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of N-isopropyl-2-methyl-5-formyl-3-pyrrole carboxylic acid ethyl ester of the present invention, its detailed steps are as follows:
[0037] a. First, dissolve D-glucosamine hydrochloride (645.2mg, 3mmol) in 15mL of water, add ethyl acetoacetate (468.5mg, 3.6mmol) and NaHCO after completely dissolving 3 (377.9mg, 4.5mmol); heated to 85°C for reflux reaction for 10h, TLC (developing solvent is a mixture of chloroform and methanol, the volume ratio of chloroform and methanol is 10:1) detection during the reaction; after the reaction , standing at 4°C for 24h, after standing, a white substance was precipitated, and then filtered under reduced pressure, and the white solid obtained after suction filtration was recrystallized with acetone to obtain a white solid compound Ia (2-methyl-5- 1'2'3'4'-tetrahydroxybutyl-3-pyrrole carboxylate ethyl ester) (663mg, 81%);
[0038] The mp of compound Ia: 153~154°C; 1 H NMR (D 2 O, 400MHz), δ: 1.21 (t, 3H, J=7.1Hz...
Embodiment 2
[0045] Embodiment 2: basically the same as Embodiment 1, the difference is:
[0046] The difference between the preparation method of N-isobutyl-2-methyl-5-formyl-3-pyrrolecarboxylic acid ethyl ester of the present invention and Example 1 is:
[0047] In step c: the compound II (0.2716g, 1.5mmol) obtained in step b was placed in a reaction vessel, and bromoisobutane (0.2466g, 1.80mmol), phase transfer catalyst tetrabutylammonium bromide (0.0223 g, 0.07mmol), solvent acetonitrile 7mL and anhydrous potassium carbonate (0.621g, 4.5mmol), heated up to 65°C in an oil bath for 8h, and carried out TLC during the reaction (the developing solvent was petroleum ether and ethyl acetate) The mixture, when petroleum ether and ethyl acetate are mixed, the volume ratio between the two is 3:1) detection, after the reaction, acetonitrile is evaporated under reduced pressure, 15mL water is added to the remaining reactant, potassium carbonate is dissolved after adding water , separate the organ...
Embodiment 3
[0051] Embodiment 3: basically the same as Embodiment 1, the difference is:
[0052] The difference between the preparation method of N-isoamyl-2-methyl-5-formyl-3-pyrrolecarboxylic acid ethyl ester of the present invention and Example 1 is:
[0053] In step c: the compound II (0.2716g, 1.5mmol) obtained in step b was placed in a reaction vessel, and bromoisopentane (0.2492g, 1.65mmol), phase transfer catalyst tetrabutylammonium bromide (0.0161 g, 0.05mmol), solvent acetonitrile 7.5mL and anhydrous potassium carbonate (0.621g, 4.5mmol), heated up to 65°C in an oil bath for 8h, and performed TLC during the reaction (developing solvents were petroleum ether and ethyl acetate The mixture, the volume ratio between the two when petroleum ether and ethyl acetate are mixed is 3:1) detection, after the reaction is over, acetonitrile is evaporated under reduced pressure, 20mL of water is added to the remaining reactant, and potassium carbonate is made after adding water Dissolved and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com