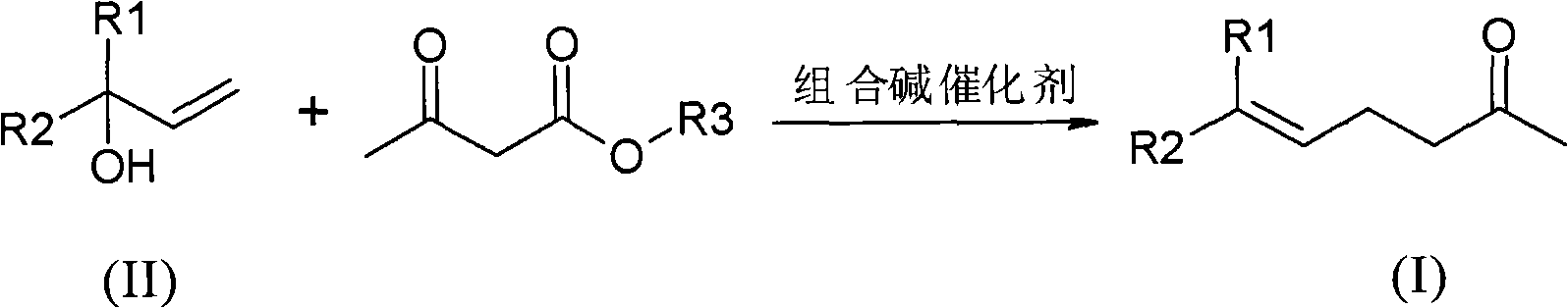
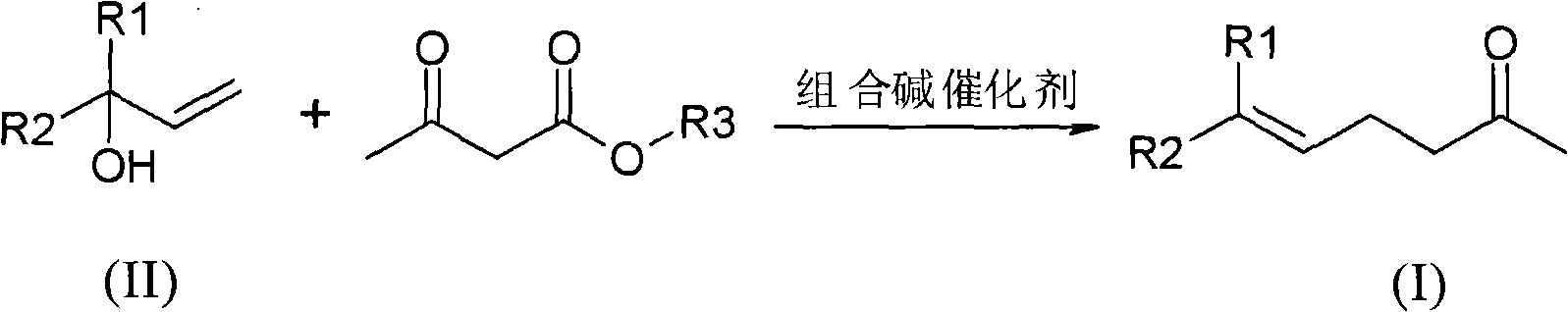
Method for preparing gamma and delta unsaturated ketone
An unsaturated, general formula technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of dangerous production operations, limited transportation, and high production costs, and achieves easy access to raw materials, The raw materials are cheap and easy to recycle and apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of geranylacetone (6,10-dimethyl-5,9-undecadien-2-one):
[0030] In a reaction vessel with a distillation device, under nitrogen protection, add linalool (100.0 g, 0.65 mol) and 5.0 g of triethanolamine solution containing 10% (mass ratio) sodium ethoxide, and stir to raise the temperature. When the temperature of the system was raised to 100° C., ethyl acetoacetate (84.5 g, 0.65 mol) was added dropwise, and the dropwise was completed within 2 hours by controlling the speed. After the dropwise addition, the temperature of the system was raised to 160° C., and the reaction was kept for 5 hours. Remove the low-boiling components generated in the reaction process, and then carry out vacuum distillation to obtain 10.0 g of the front fraction at 25-55 ° C / 50 Pa; 115.1 g of the main fraction (geranyl acetone) at 55-65 ° C / 50 Pa. The yield was 91.3%, and the purity detected by GC was 97.5%.
Embodiment 2
[0032] Preparation of Geranylacetone
[0033] In a reaction vessel with a distillation device, under nitrogen protection, add linalool (100.0 g, 0.65 mol) and 10.0 g of triethanolamine solution containing 5% (mass ratio) sodium methoxide, and stir to raise the temperature. When the temperature of the system was raised to 150° C., methyl acetoacetate (86 g, 0.74 mol) was added dropwise, and the dropwise was completed within 3 hours at a controlled rate. After the dropwise addition, the temperature of the system was raised to 190° C. and kept for 8 hours. The low-boiling components generated during the reaction were removed, and distillation under reduced pressure was started. Obtain 13.5g of the front fraction at 25-55°C / 50Pa; 113.2g of the main fraction (geranylacetone) at 55-65°C / 50Pa, with a yield of 89.7% and a purity of 98.5%.
Embodiment 3
[0035] The preparation of geranyl acetone (combined alkali recovery applies mechanically):
[0036] Under the protection of nitrogen, in the reaction vessel left with the distillation raffinate in Example 1, add (100.0 g, 0.65 mol) linalool and 10.0 g of the fraction before distillation, and stir to raise the temperature. When the temperature of the system was raised to 100° C., ethyl acetoacetate (89 g, 0.68 mol) was added dropwise at a controlled rate within 2 hours. After the dropwise addition, the temperature of the system was raised to 180° C. and kept for 6 hours. The low-boiling components generated during the reaction were removed, and distillation under reduced pressure was started. Obtain 10.2g of the front fraction at 25-50°C / 50Pa; 118.1g of the main fraction at 50-80°C / 50Pa, with a yield of 93.7% and a purity of 97.6%.
[0037] The same method was applied mechanically 7 times again, and the yield and purity of the main fraction are shown in Table 1.
[0038] Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com