Method for biological catalysis of unsymmetrical reduction carbon based compound in water/ion liquid diphasic system
A carbonyl compound, ionic liquid technology, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of low conversion rate and product concentration, difficulty in separation and recovery, microbial cytotoxicity, etc., and achieve conversion rate. and optical purity promotion, product separation is simple, and the effect of increasing product concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Choice of ionic liquid
[0034] A. Selection of Ionic Liquids in Aureobasidium pullulans CGMCC No.1244 Asymmetric Reduction of COBE to Form (S)-CBHE
[0035] Slant culture: the medium is 100mL wort, add 2g glucose, 2g agar, pH6.5, sterilize at 121°C for 20 minutes according to the usual method, cool down after sterilization to inoculate the slant, cultivate at 30°C for 2 days, and use it as an activated seed on the slant .
[0036] Seed cultivation and fermentation: medium: maltose 30g / L, yeast extract 20g / L, peptone 5g / L, (NH 4 ) 2 SO 4 5g / L, KH 2 PO 4 2g / L, MgSO 4 ·7H 2 O 0.7g / L, pH 6.0. The filling volume is 80mL of 500mL Erlenmeyer flask, sterilized at 121°C for 20 minutes, cooled and connected to slanted seeds after sterilization, shaken at 180 rpm, and cultivated at 30°C for 2 days, as seeds or fermentation enzyme liquid (whole cell catalyst).
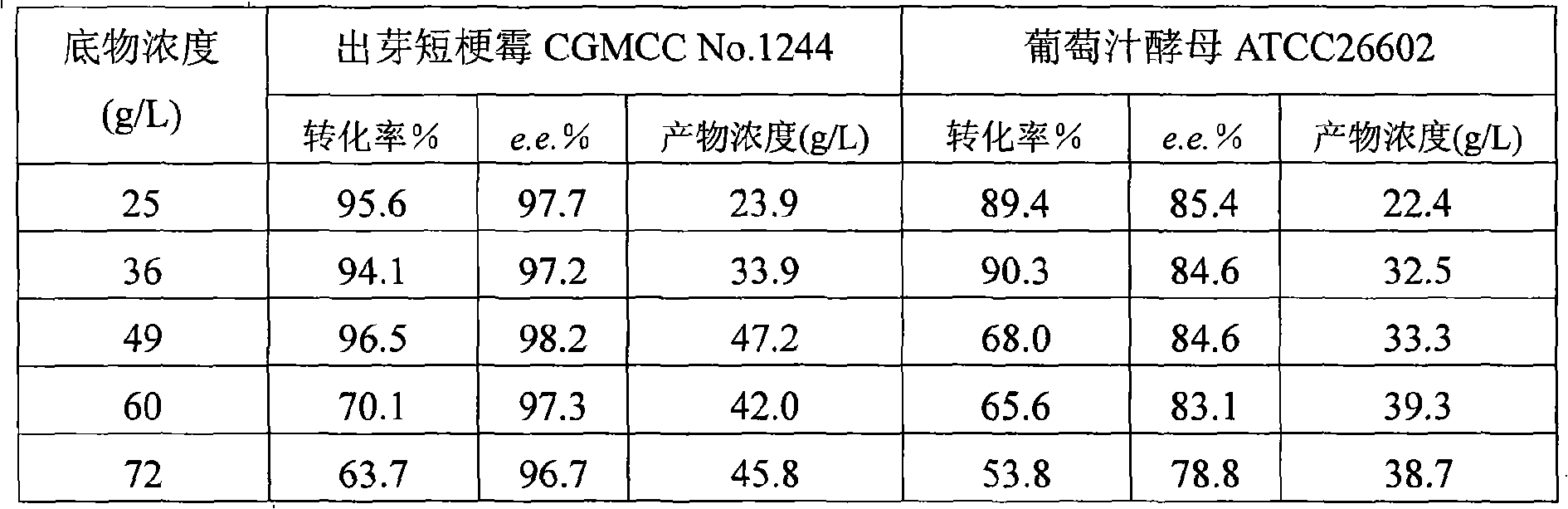
[0037] The content of wet cells in the fermented enzyme liquid is 2.5g / 100mL, centrifuge for 10 minutes (8,000 ...
Embodiment 2
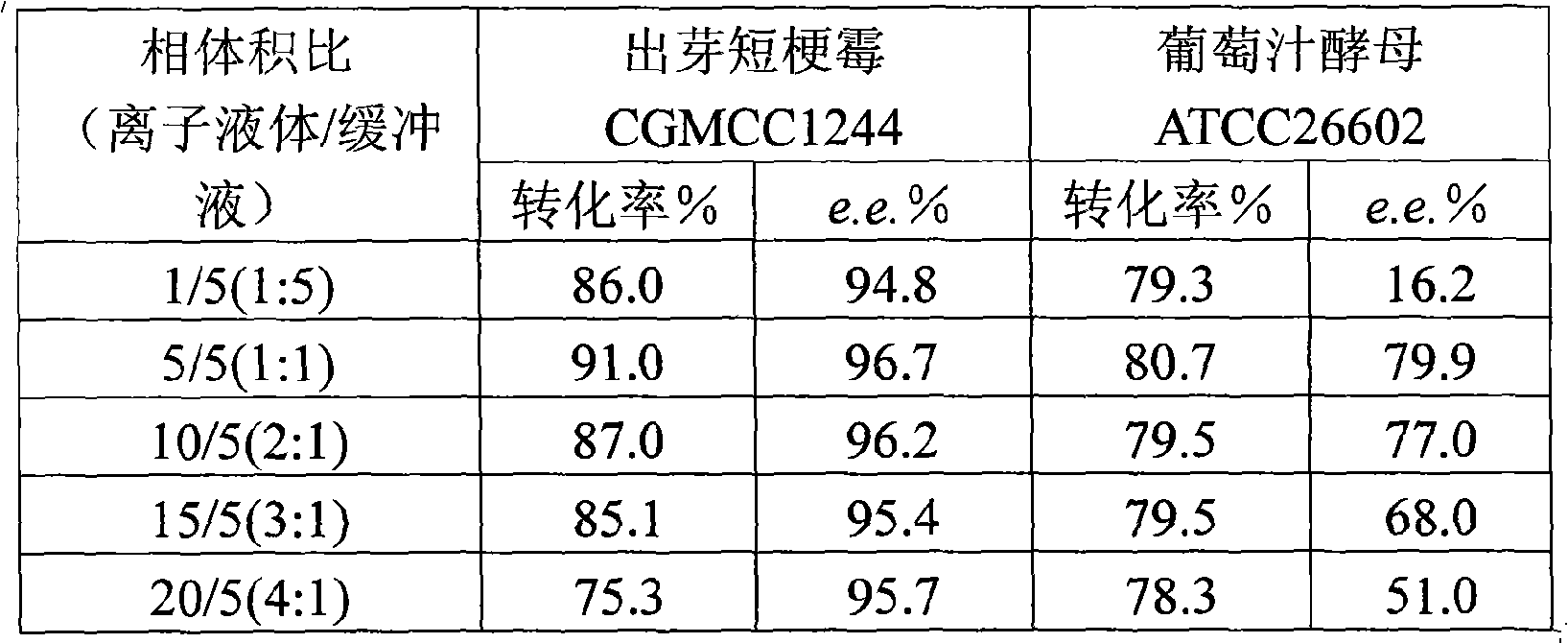
[0049] Effect of different phase volume ratios on transformation reactions in water / ionic liquid two-phase system
[0050] After Aureobasidium pullulans CGMCC No.1244 or grape juice yeast ATCC 26602 were cultured for 24 hours to produce enzyme according to the method in Example 1, weigh 1 g of wet bacteria and add 10 mL of 0.1 mol / L, pH 6.6 to 7.0 potassium phosphate buffer in a 150 mL Erlenmeyer flask , the substrate content is 30g / L, and the ionic liquid [bmim]PF with different phase volume ratios are added respectively 6 , the volume ratio of the ionic liquid to the buffer solution was 1:5, 5:5, 10:5, 15:5, 20:5, and the reaction was carried out at 30° C. and 180 rpm for 8 hours. After the reaction, the reaction solution was centrifuged to remove the bacteria and the water phase to obtain the ionic liquid phase, which was extracted three times with an equal volume of isopropanol, the combined extracts were dried with an appropriate amount of anhydrous magnesium sulfate, fil...
Embodiment 3
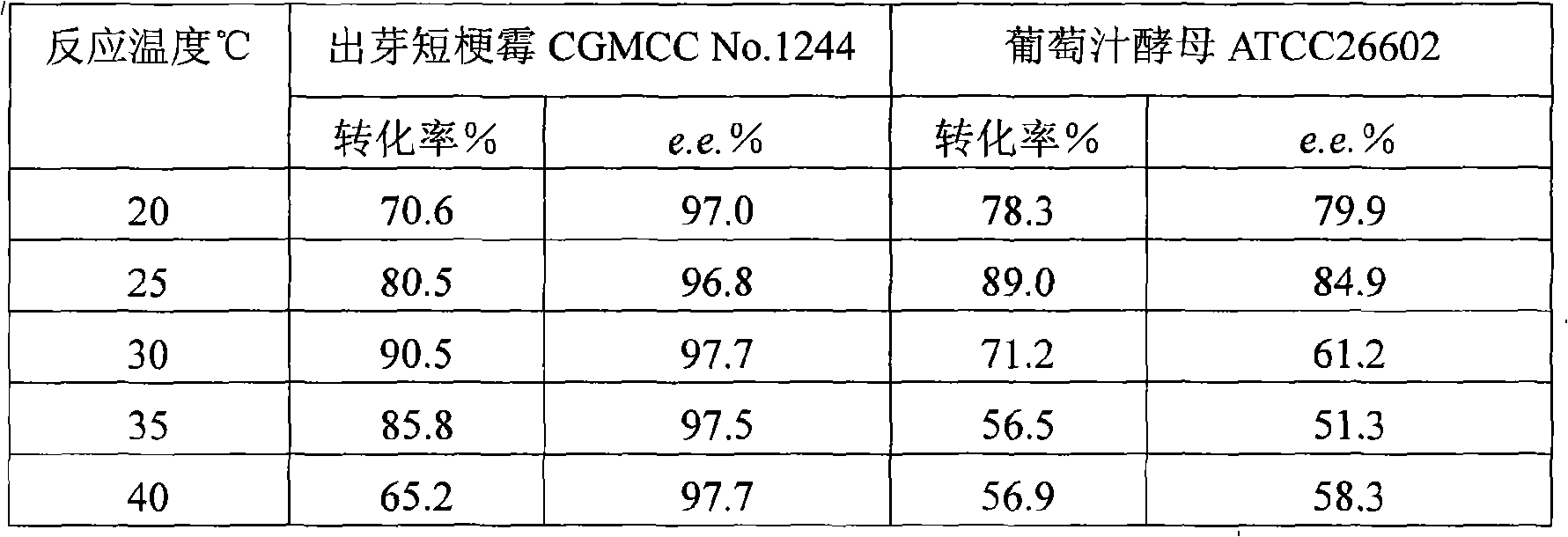
[0055] Effects of Different Temperatures on Transformation Reactions in Water / Ionic Liquid Two-Phase System
[0056] After Aureobasidium pullulans CGMCC No.1244 or grape juice yeast ATCC 26602 were cultured for 24 hours according to the method of Example 1, 1 g of wet bacteria was weighed and added to 5 mL of 0.1 mol / L, pH 6.6-7.0 potassium phosphate buffer in a 50 mL Erlenmeyer bottle In, with an equal volume of ionic liquid [bmim]PF 6 Mixed, the substrate content was 35g / L, and the effects of different temperatures on the conversion reaction were investigated respectively. The reaction was carried out at 20°C, 25°C, 30°C, 35°C, 40°C and 180 rpm for 8 hours. After the reaction, the reaction solution was centrifuged to remove the bacteria and the water phase to obtain an ionic liquid phase, which was extracted three times with an equal volume of isopropanol, and the extracts were combined, dried with an appropriate amount of anhydrous magnesium sulfate, filtered and then analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com