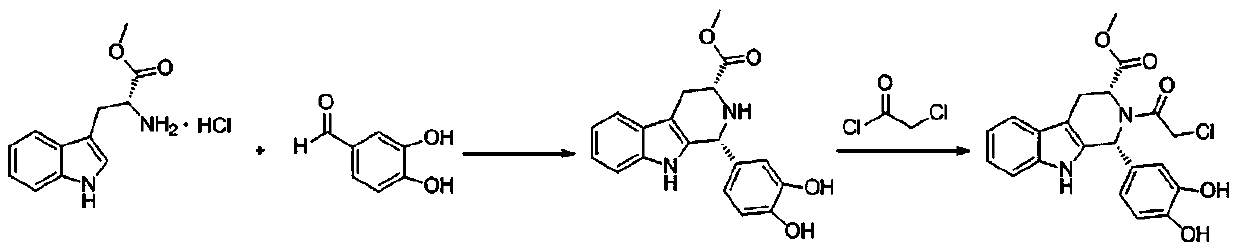
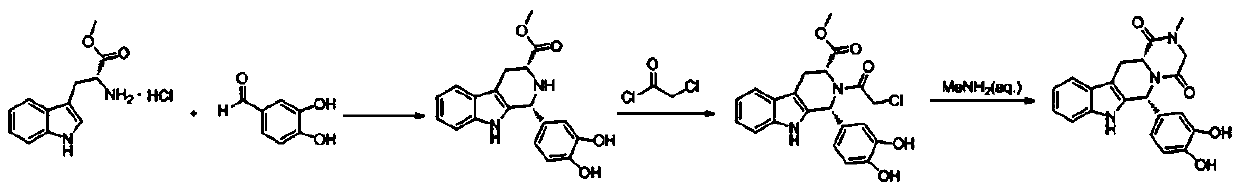
The synthetic method of tadalafil
A technique for the synthesis of tadalafil, which is applied in the field of organic synthesis, can solve the problems of low yield, cumbersome routes, harsh reaction conditions, etc., and achieve the effects of simple treatment, easy recovery and application, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
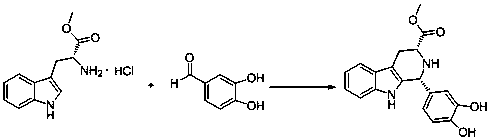
[0047] Add 25.5 grams of D-tryptophan methyl ester hydrochloride to the reaction flask, add 150 milliliters of isopropanol at room temperature, stir, add 1.2 equivalents of 3,4-dihydroxybenzaldehyde, and react at 85°C for 8 hours until the raw material The reaction was basically complete, cooled to room temperature, filtered, and the solid was washed with 50 ml of isopropanol, and then dried in an oven to constant weight. 34.6 g of compound I were obtained with a yield of 92%. The synthetic route map is attached figure 1 shown.
Embodiment 2
[0049] Add 25.5 grams of D-tryptophan methyl ester hydrochloride to the reaction flask, add 250 milliliters of propionitrile at room temperature, stir, add 1.5 equivalents of 3,4-dihydroxybenzaldehyde, and react at 85°C for 16 hours until the raw materials are basically After the reaction was complete, it was cooled to room temperature, filtered, and the solid was washed with 50 ml of isopropanol, and then dried in an oven to constant weight. 34.2 g of compound I were obtained with a yield of 97%. The synthetic route map is attached figure 1 shown.
Embodiment 3
[0051] Add 25.5 grams of D-tryptophan methyl ester hydrochloride to the reaction flask, add 375 milliliters of methanol at room temperature, stir, add 1.5 equivalents of 3,4-dihydroxybenzaldehyde, and react at 60°C for 24 hours until the raw materials basically react completely, cooled to room temperature, filtered, the solid was washed with 50 ml of methanol, and then dried in an oven to constant weight. 33.8 g of compound I were obtained with a yield of 90%. The synthetic route map is attached figure 1 as shown..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com