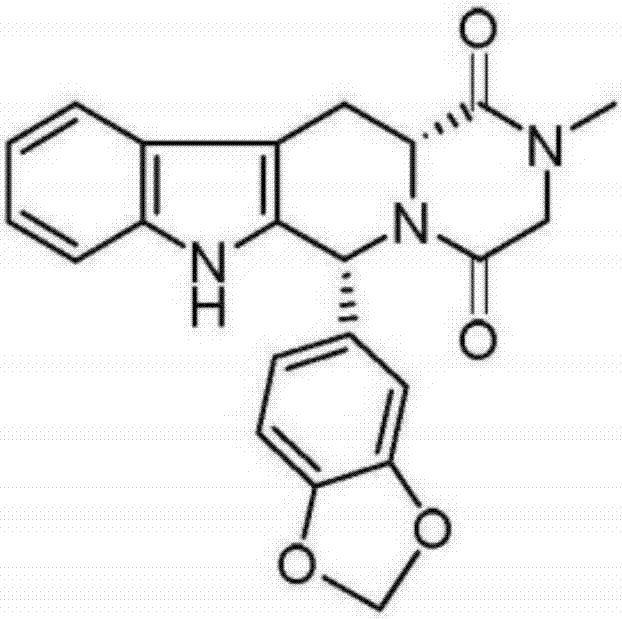
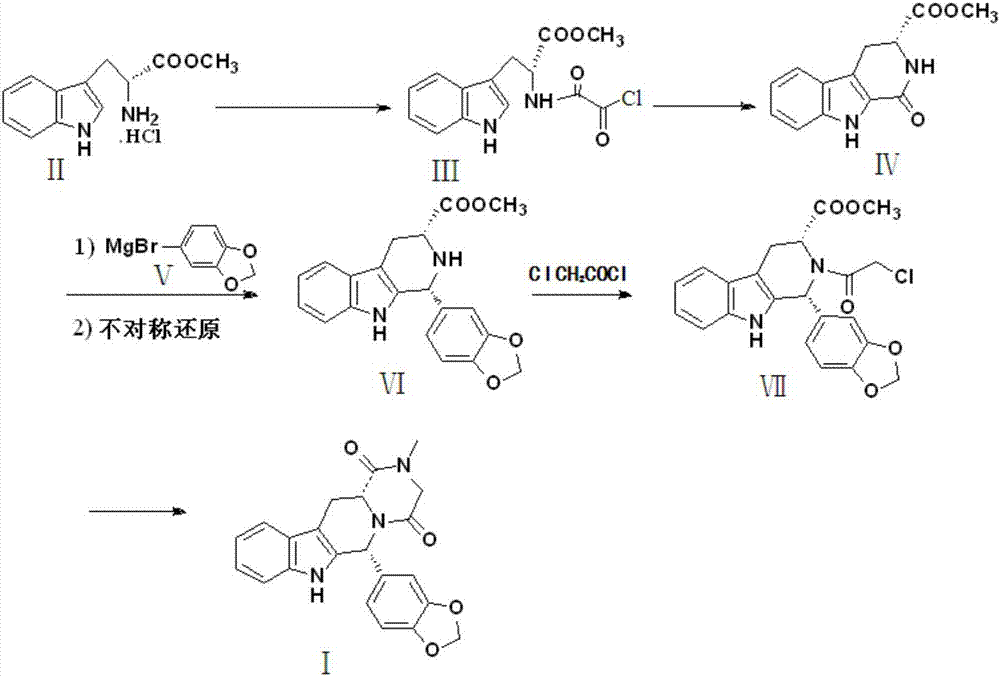
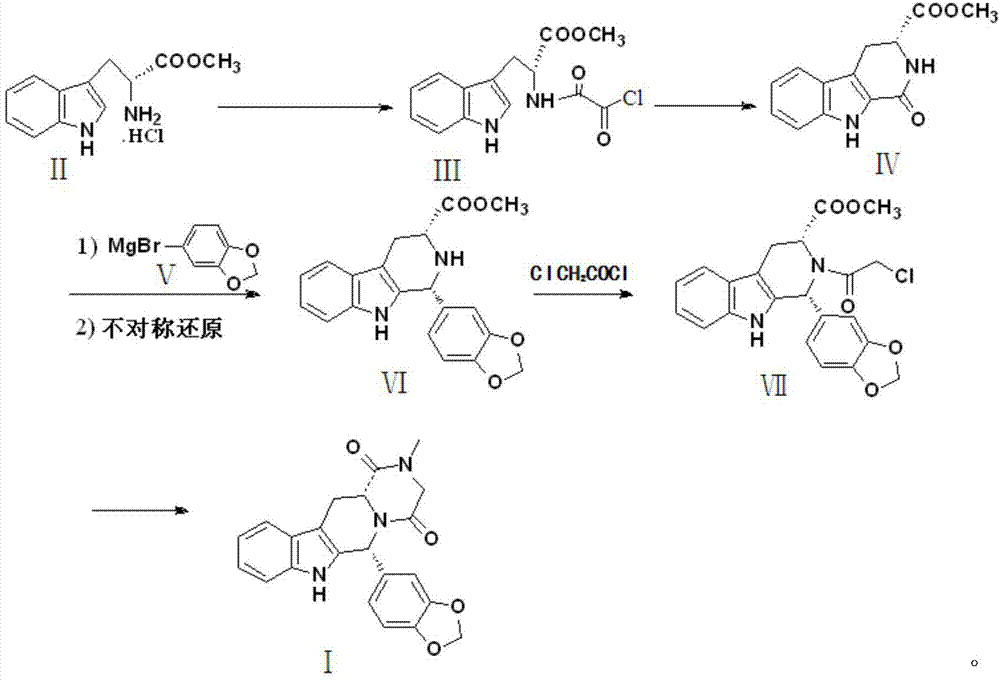
Preparation method of tadalafil
A technology of tadalafil and compounds, which is applied in the field of drug synthesis, can solve problems such as trans isomers, and achieve the effects of high total yield, stable process, and simple overall route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of compound Ⅲ
[0031] Add 25.47g of D-tryptophan methyl ester hydrochloride, 15.82g of pyridine, and 200ml of ethyl acetate into the reaction vessel in sequence, stir to dissolve, keep the temperature in an ice bath at 0-5°C, and slowly add dropwise the solution containing 11.42g of oxalyl chloride 20ml of ethyl acetate solution, stirred for 2h to complete the reaction, washed 3 times with distilled water (200ml×3) after the reaction, added sodium sulfate to dry, distilled off the solvent, and crystallized with 95% ethanol to obtain 21.44g of compound III with a yield of 82%. 99.90% pure.
Embodiment 2
[0033] Preparation of compound Ⅲ
[0034] Add 25.47g of D-tryptophan methyl ester hydrochloride, 36.65g of 4-dimethylaminopyridine, and 200ml of dichloromethane in turn into the reaction vessel, stir to dissolve, keep the temperature in an ice bath at 0-5°C, and slowly add the 11.42 g of oxalyl chloride in 20 ml of dichloromethane solution, stirred for 2 hours to complete the reaction, washed 3 times with distilled water (200 ml × 3) after the reaction, added sodium sulfate to dry, distilled off the solvent, crystallized with 95% ethanol to obtain 21.96 g of compound III, Yield 84%, purity 99.93%.
Embodiment 3
[0036] Preparation of compound Ⅲ
[0037] Add 25.47g of D-tryptophan methyl ester hydrochloride, 30.35g of N-methylmorpholine, and 200ml of ethyl acetate into the reaction vessel in sequence, stir to dissolve, keep the temperature in an ice bath at 0-5°C, and slowly add the 11.42 g of oxalyl chloride in 20 ml of ethyl acetate solution, stirred for 2 h to complete the reaction, washed 3 times with distilled water (200 ml × 3), added sodium sulfate to dry, distilled off the solvent, crystallized with 95% ethanol to obtain 25.26 g of compound III, Yield 87%, purity 99.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com